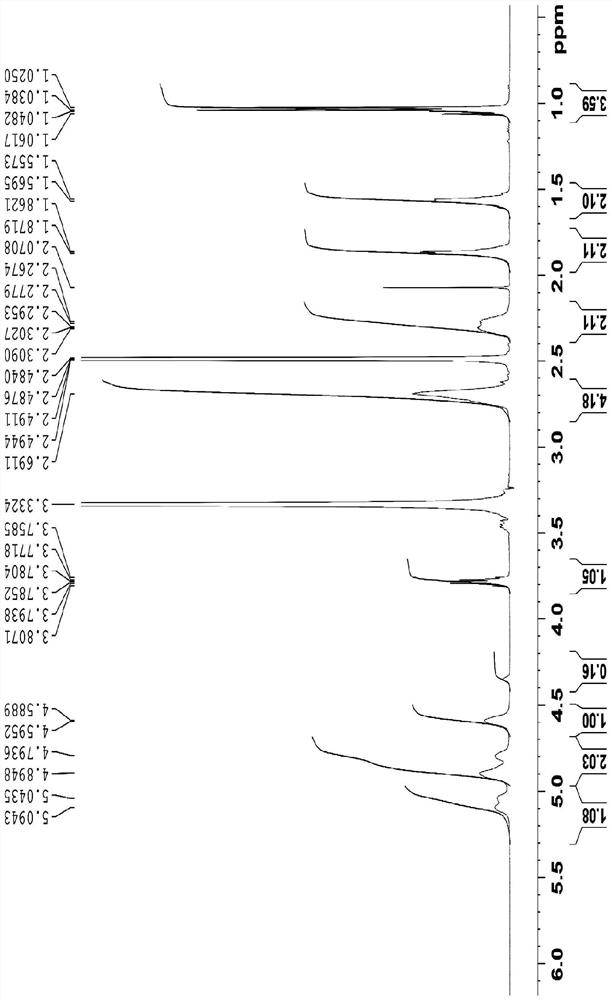
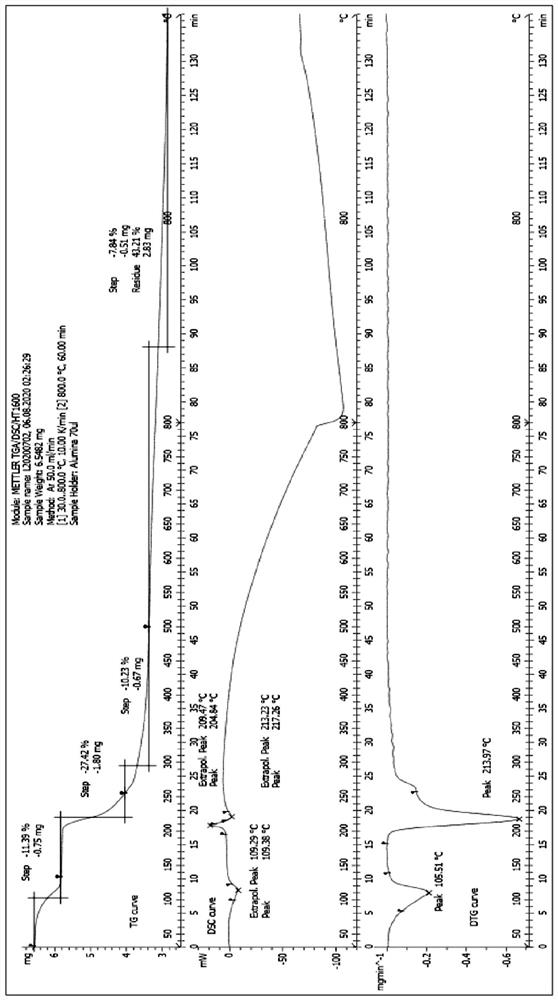
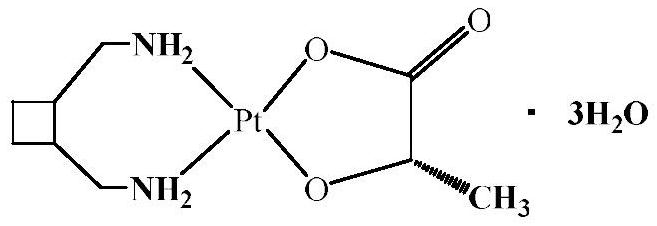
Preparation method of lobaplatin trihydrate
A technology of lobaplatin trihydrate and aqueous solution, which is applied in the field of preparation of lobaplatin trihydrate, which can solve the problems of no protection measures for acetone, increased requirements for filtration equipment, and increased production costs, so as to reduce impurity intake and shorten concentration Time, time-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The present invention will be described in further detail below in conjunction with the examples, but the protection scope of the present invention is not limited to the content described. Embodiment 1, a kind of preparation method of lobaplatin trihydrate, concrete operation is as follows:
[0039] (1) Potassium iodoplatinite synthesis process
[0040] Add 10.0 g of potassium chloroplatinite to water for dissolution, and another potassium iodide with a molar ratio of 1:5 to potassium chloroplatinite is added to water for dissolution. The two solutions are mixed and stirred at 20°C for 2.0 hours in the dark. Wherein, potassium chloroplatinite: water is 1g: 1mL, potassium iodide: water is 1g: 1mL, and the dissolution temperature is 40°C.
[0041] (2) Synthesis process of cis-diiodo-(trans-1,2-diaminomethyl-cyclobutane) platinum(Ⅱ) crude product
[0042] Weigh trans-1,2-diaminomethyl-cyclobutane hydrochloride according to the molar ratio of potassium chloroplatinite to ...
Embodiment 2
[0059] Embodiment 2, a kind of preparation method of lobaplatin trihydrate, concrete operation is as follows:
[0060] (1) Potassium iodoplatinite synthesis process
[0061] Add 100.0 g of potassium chloroplatinite to water for dissolution, and another potassium iodide with a molar ratio of 1:6 to potassium chloroplatinite is added to water for dissolution. The two solutions are mixed and stirred for 1.0 h at 30°C in the dark. Wherein, potassium chloroplatinite: water is 1g: 5mL, potassium iodide: water is 1g: 3mL, and the dissolution temperature is 50°C.
[0062] (2) Synthesis process of cis-diiodo-(trans-1,2-diaminomethyl-cyclobutane) platinum(Ⅱ) crude product
[0063] Weigh trans-1,2-diaminomethyl-cyclobutane hydrochloride according to the molar ratio of potassium chloroplatinite to trans-1,2-diaminomethyl-cyclobutane hydrochloride 1:1.5 , Weigh potassium hydroxide according to the molar ratio of trans-1,2-diaminomethyl-cyclobutane hydrochloride to potassium hydroxide 1:1...
Embodiment 3
[0079] Embodiment 3, a kind of preparation method of lobaplatin trihydrate, concrete operation is as follows:
[0080] (1) Potassium iodoplatinite synthesis process
[0081] Add 1000.0 g of potassium chloroplatinite to water for dissolution, and another potassium iodide with a molar ratio of 1:8 to potassium chloroplatinite is added to water for dissolution. The two solutions are mixed and stirred at 40°C in the dark for 0.5 h. Wherein, potassium chloroplatinite: water is 1g: 10mL, potassium iodide: water is 1g: 5mL, and the dissolution temperature is 60°C.
[0082] (2) Synthesis process of cis-diiodo-(trans-1,2-diaminomethyl-cyclobutane) platinum(Ⅱ) crude product
[0083] Weigh trans-1,2-diaminomethyl-cyclobutane hydrochloride according to the molar ratio of potassium chloroplatinite to trans-1,2-diaminomethyl-cyclobutane hydrochloride 1:1.8 , Weigh sodium oxide according to the molar ratio of trans-1,2-diaminomethyl-cyclobutane hydrochloride to sodium oxide 1:1.8, accordin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com