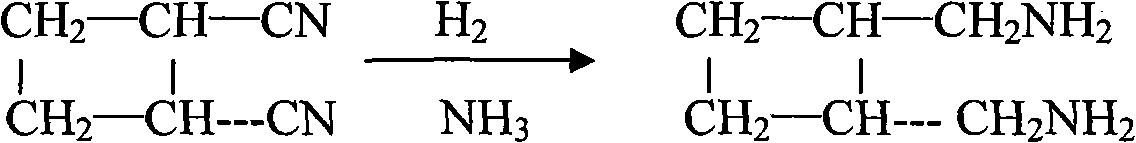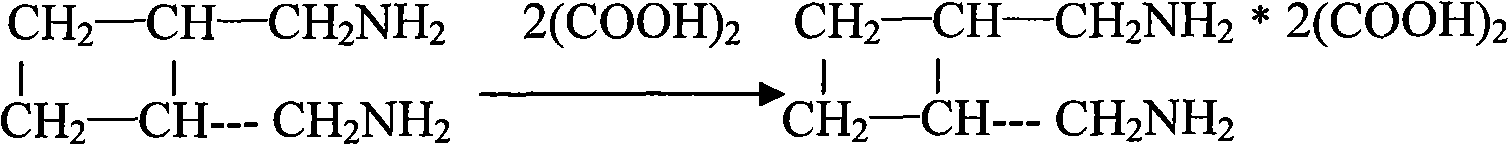Preparation method of diaminomethyl cyclobutane oxalate
A technology of diaminomethyl cyclobutane and aminomethyl cyclobutane is applied in the field of preparation of diaminomethyl cyclobutane oxalate, and can solve the problem of affecting the lobaplatin synthesis reaction speed, product yield and purity, and reverse reaction. Formula-diaminomethylcyclobutane has problems such as unstable performance, limited product yield and purity, etc., to achieve the effects of easy storage and transportation, stable properties, and easy operation to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Take 200ml of acrylonitrile, add 0.05g of Fecl3 and 0.044g of p-methoxyphenol at a temperature of 215°C and pressurize nitrogen to 17bar, and mix and react for 8 hours. After the reaction is complete, 20ml of dark red liquid is obtained. After distillation at 175°C, 12g of crude dicyanocyclobutane was obtained, with a cis-to-trans ratio of about 4:6, and then the crude product was rectified at 150°C to obtain 7.5g of trans-dicyanocyclobutane, with a melting point of 37.5°C;
[0030] (2), after feeding ammonia gas into the trans-dicyanocyclobutane, the temperature rises to 80°C, the hydrogen gas is pressurized to 20bar, catalyzed by active nickel, the reaction time is 20h, and 16ml of light yellow liquid is obtained, that is, trans Formula - diaminomethylcyclobutane;
[0031] 3. Stir 16ml of trans-diaminomethylcyclobutane and 3.5g of anhydrous oxalic acid at 70°C under normal pressure for 3.5h, combine to form 3.3g of crude diaminomethylcyclobutane oxalate, and then ...
Embodiment 2
[0033] 1. Take 100ml of acrylonitrile, add 0.03g of Fecl3 and 0.025g of p-methoxyphenol at a temperature of 200°C and pressurize nitrogen to 17bar, and mix and react for 6 hours. After the reaction is complete, 10ml of dark red liquid is obtained. After distillation, 6.3 g of crude dicyanocyclobutane was obtained, with a cis-to-trans ratio of about 4:6, and then the crude product was subjected to rectification at 140°C to obtain 4 g of trans-dicyanocyclobutane, with a melting point of 37.5°C;
[0034] 2. After passing 4 g of trans-dicyanocyclobutane through ammonia gas, the temperature rose to 85°C, the hydrogen was pressurized to 20 bar, catalyzed by active nickel, the reaction time was 15 hours, and 8.4 ml of light yellow liquid was obtained, namely trans- Diaminomethylcyclobutane;
[0035] 3. Stir 8.4ml of trans-diaminomethylcyclobutane and 2g of anhydrous oxalic acid at 65°C under normal pressure for 5h, combine to form 1.7g of crude diaminomethylcyclobutane oxalate, and t...
Embodiment 3
[0037] 1. Take 150ml of acrylonitrile, add 0.038g of Fecl3 and 0.033g of p-methoxyphenol at a temperature of 235°C and pressurize nitrogen to 17bar, and mix and react for 9 hours. After the reaction is complete, 15ml of dark red liquid is obtained. After distillation, 9.2 g of crude dicyanocyclobutane was obtained, with a cis-to-trans ratio of about 4:6. Then, the crude product was subjected to rectification at 160°C to obtain 6.2 g of trans-dicyanocyclobutane, with a melting point of 37.5°C;
[0038] 2. After passing 6.2 g of trans-dicyanocyclobutane through ammonia gas, the temperature rose to 70°C, the hydrogen gas was pressurized to 20 bar, catalyzed by active nickel, the reaction time was 25 hours, and 11.7 ml of light yellow liquid was obtained, that is, trans - Diaminomethylcyclobutane;
[0039] 3. Stir 11.7ml of trans-diaminomethylcyclobutane and 2.5g of anhydrous oxalic acid at 80°C under normal pressure for 3h, combine to form 2.5g of crude diaminomethylcyclobutane o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com