Patents
Literature
190results about How to "Not easy to deliquescence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
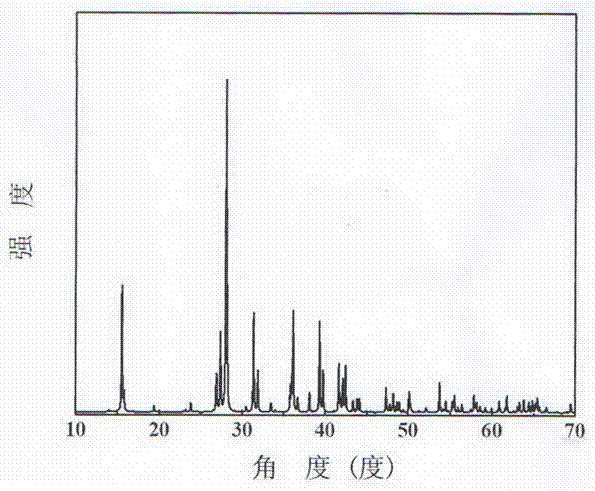
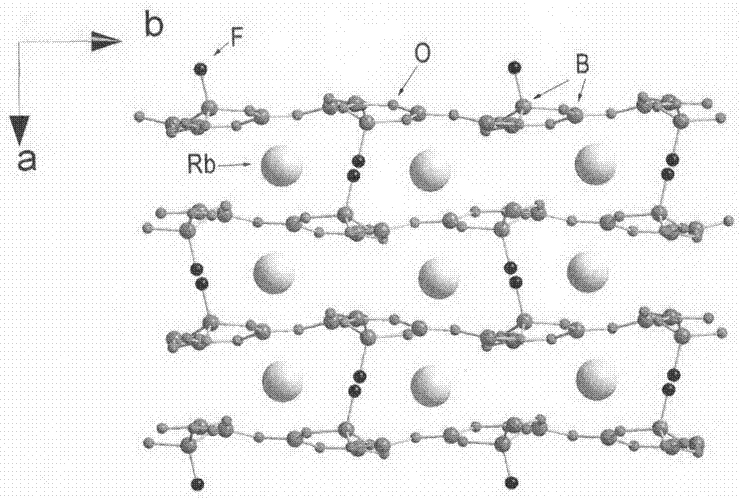
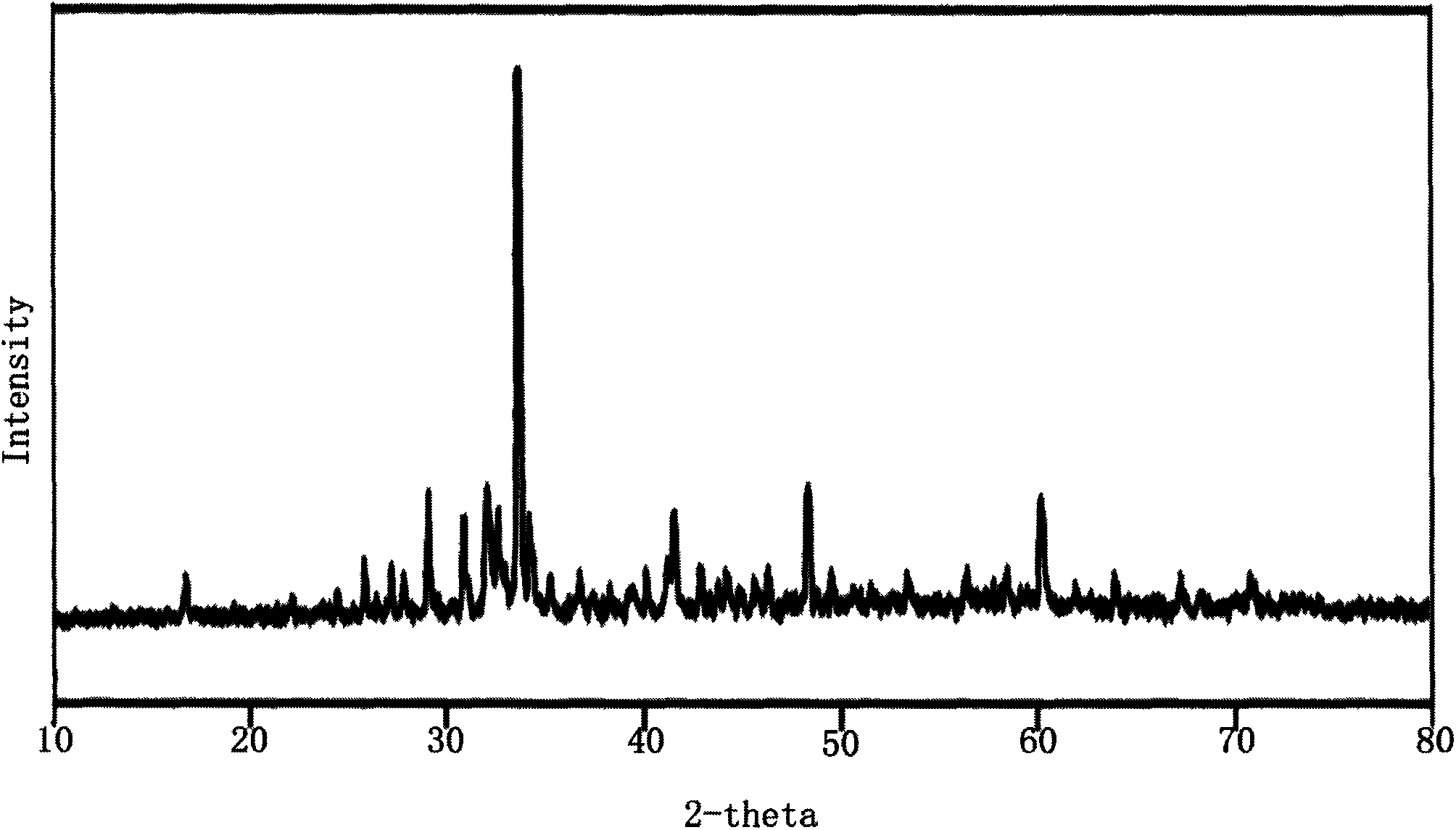
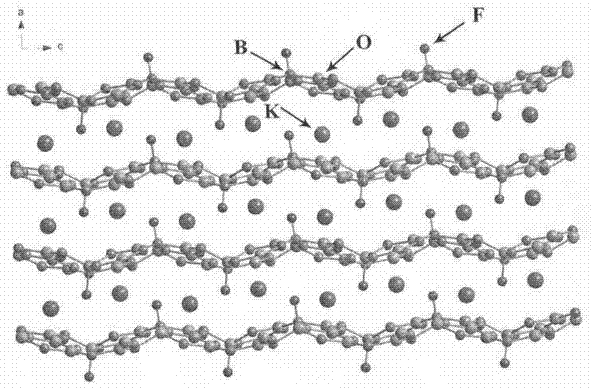
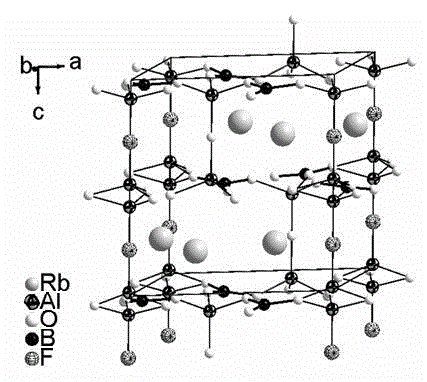
Compound rubidium borofluoride and rubidium borofluoride non-linear optical crystal and preparation method and use of rubidium borofluoride non-linear optical crystal
ActiveCN107265473ALong growth cycleEasy to getPolycrystalline material growthFrom normal temperature solutionsNonlinear optical crystalSpace group
The invention provides a compound rubidium borofluoride and a rubidium borofluoride non-linear optical crystal and a preparation method and use of the rubidium borofluoride non-linear optical crystal. The compound rubidium borofluoride has a chemical formula of RbB4O6F, has molecular weight of 243.71 and is prepared through a solid phase synthesis method or a vacuum packaging method. The crystal has a chemical formula of RbB4O6F, has molecular weight of 243.71, belongs to the orthorhombic system, and has a space group of Pna 21 and cell parameters such as a=7.6742A, b=11.228A, c=6.6218A, alpha=beta=gamma=90 degrees, unit-cell volume of 570.57 A<3>, crystal frequency-doubled effect about 1.9 times that of KH2PO4 (KDP) and ultraviolet absorption edge shorter than 190nm. The crystal is formed through a melt method, a high temperature melt method, a vacuum encapsulation method, a hydrothermal method or a room-temperature solution method. The crystal has good chemical stability and can be used as an ultraviolet and deep-ultraviolet non-linear optical crystal in an all-solid-state laser.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
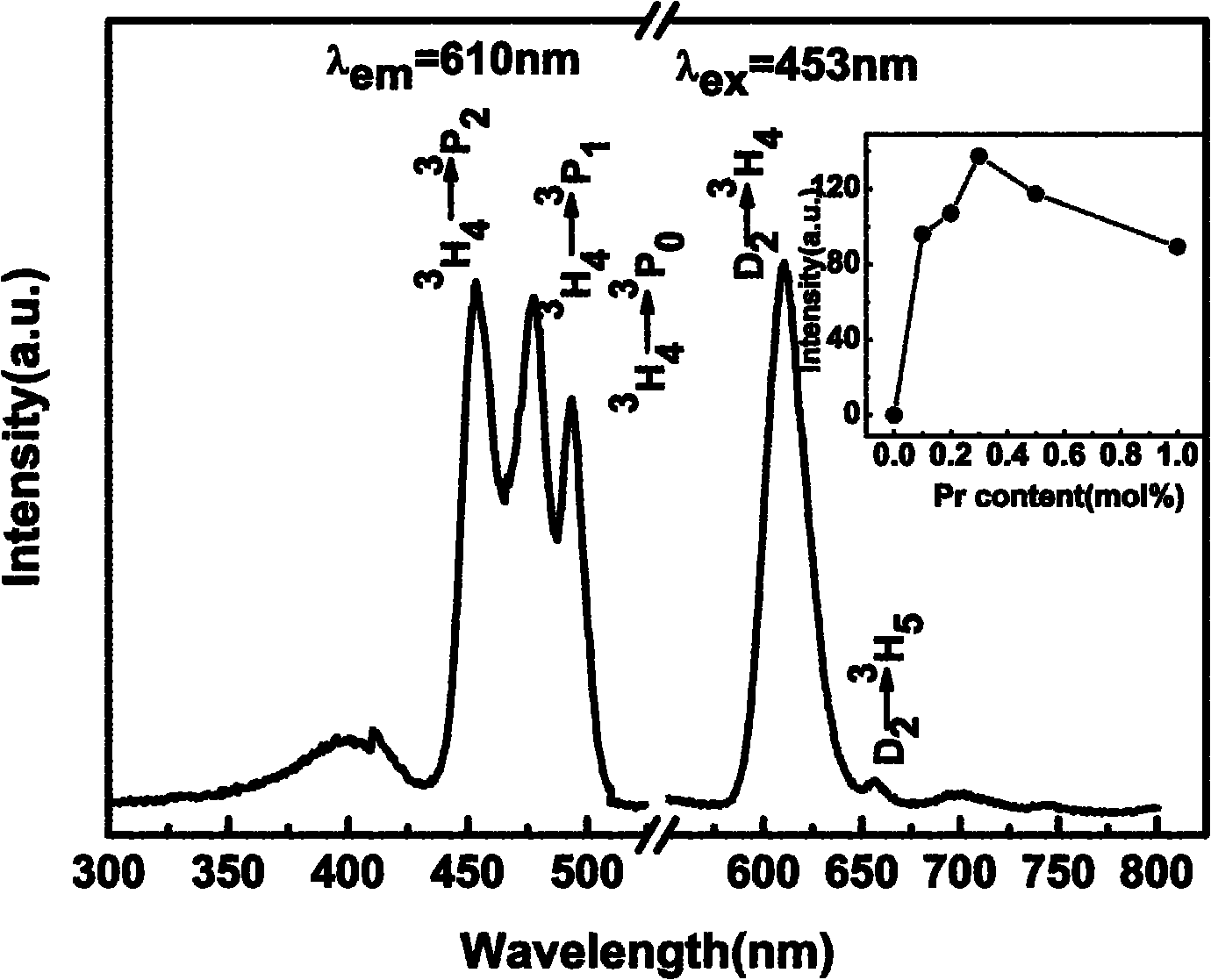
Blue-excited red fluorescent material and preparation method thereof
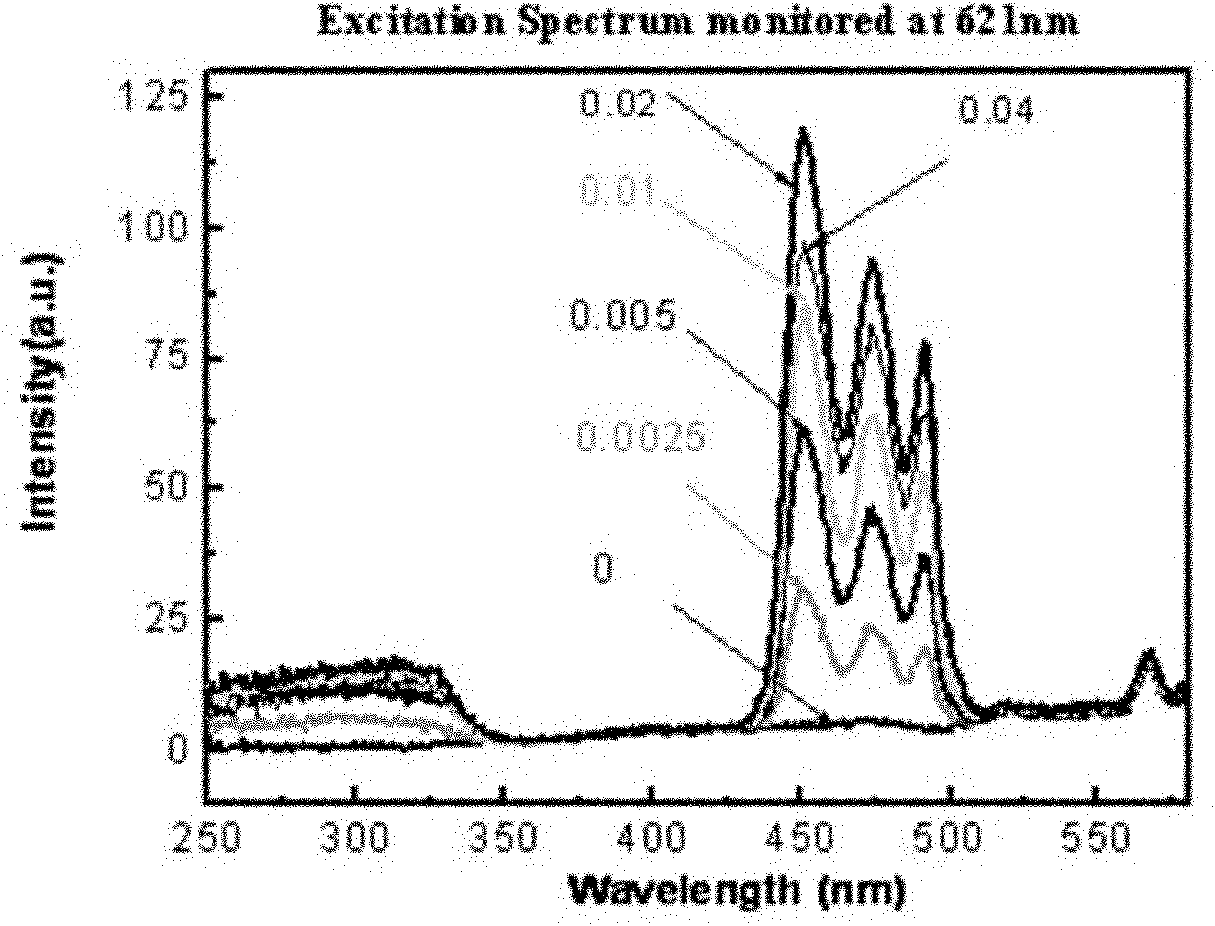
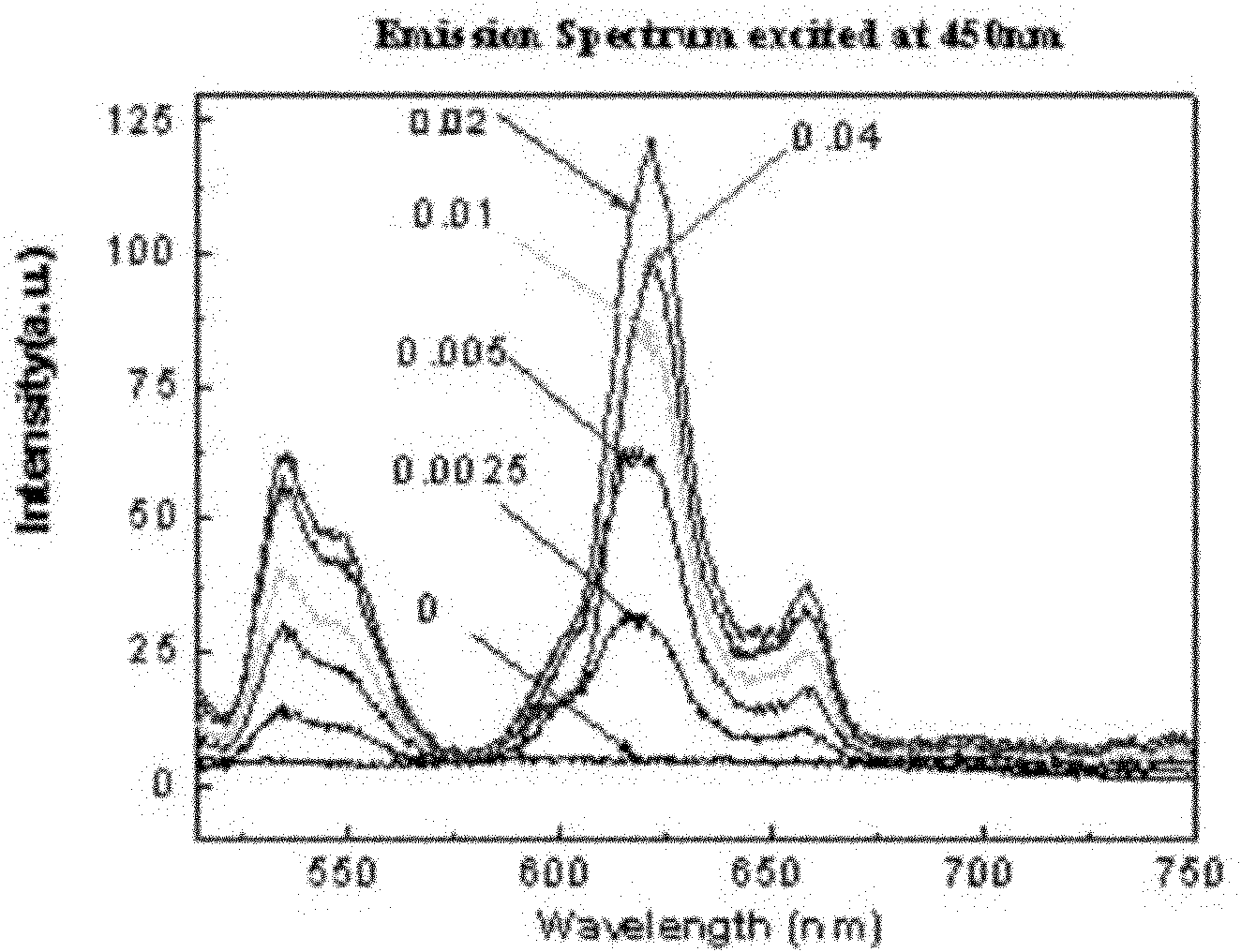
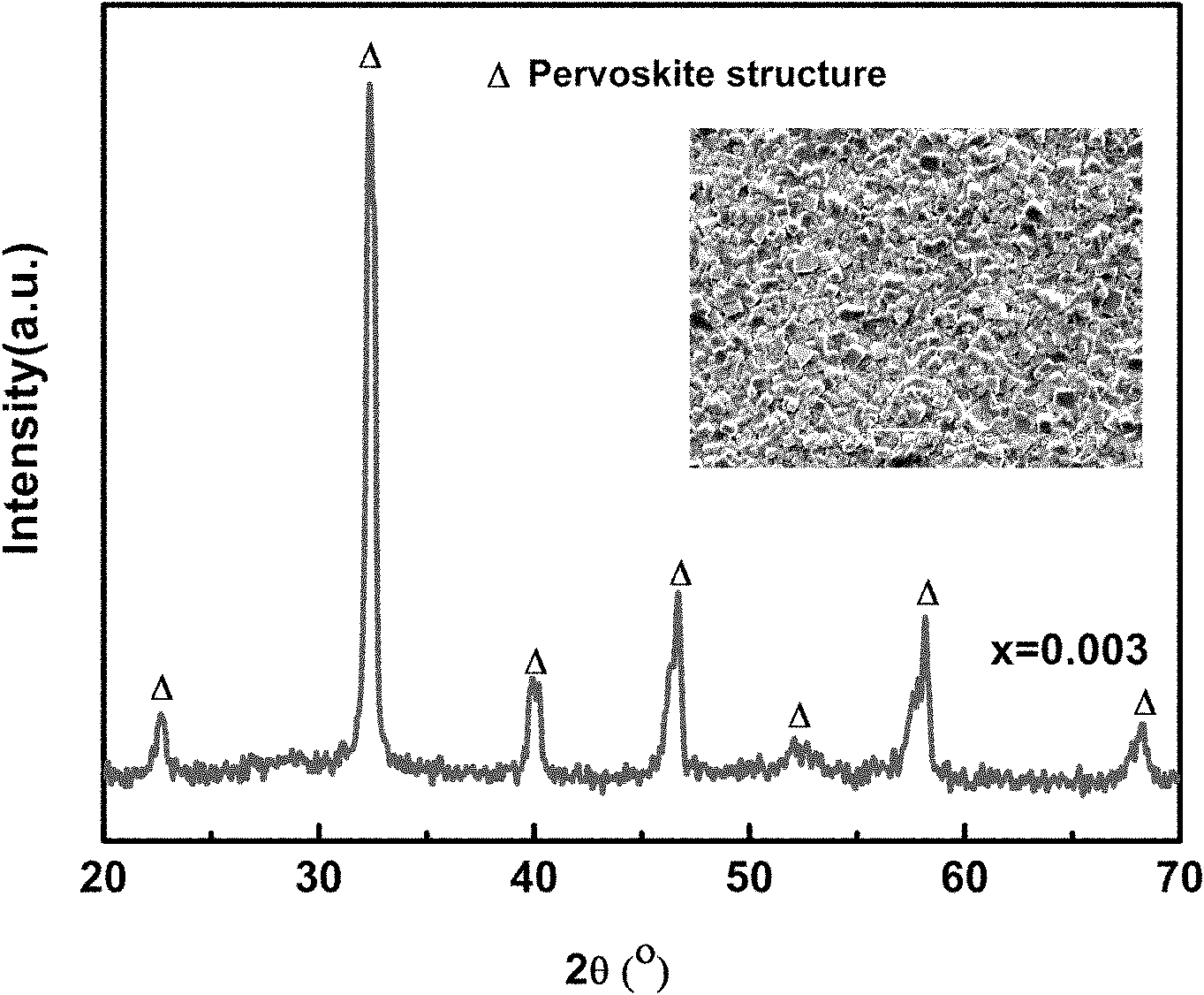
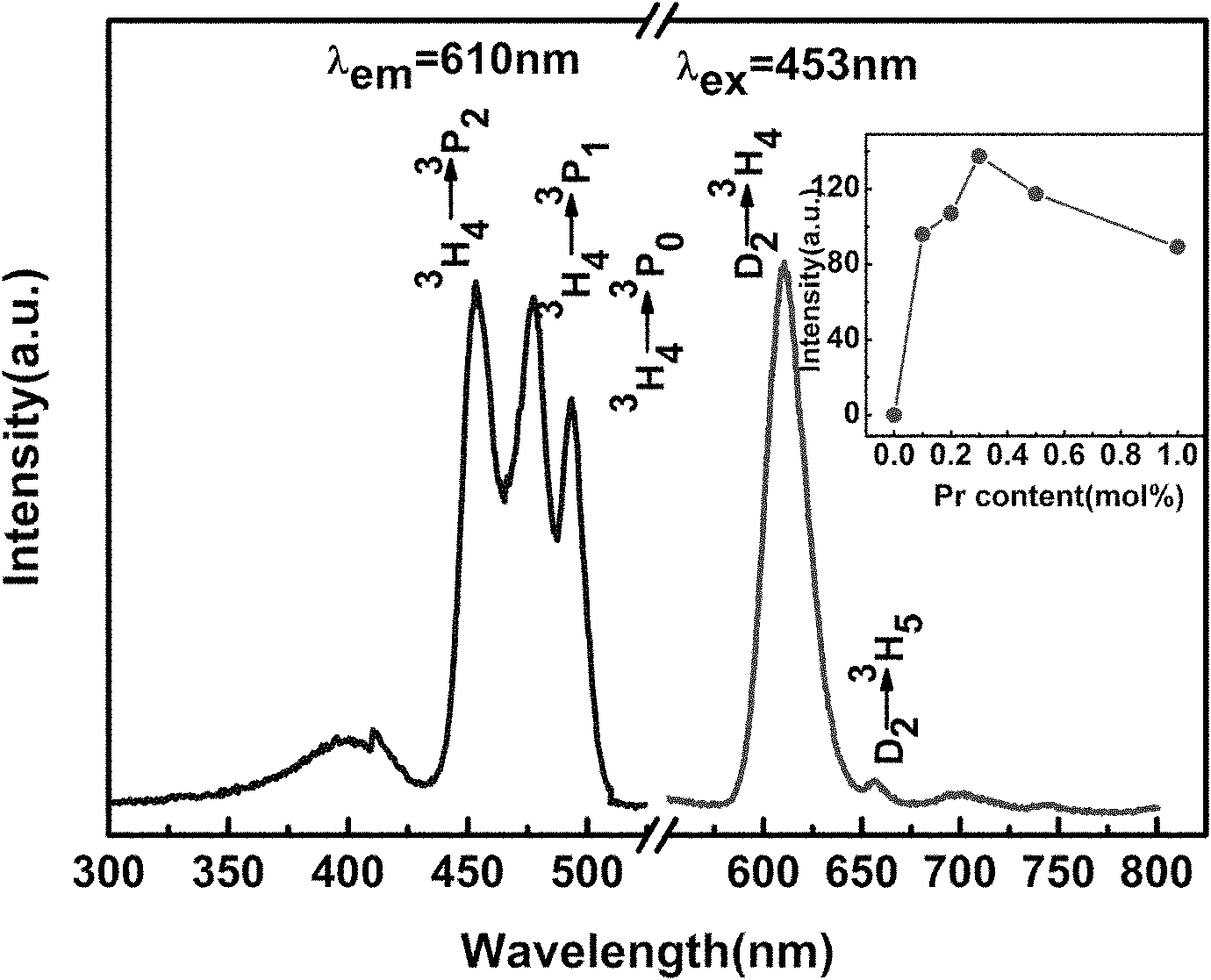
InactiveCN101974331AImprove stabilityNot easy to deliquescenceGas discharge lamp usageLuminescent compositionsRare-earth elementElectromechanics
The invention relates to a blue-excited red fluorescent material and a preparation method thereof, belonging to the field of luminescent materials. The blue-excited red fluorescent material has the following chemical expression: Ca1+d-x-mAmRxBi2Ta2-nBnO9:fC, wherein the R is selected from one or more rare earth elements of Pr, Sm, Eu and Lu; the A is selected from one or more than one of Sr, Ba, Mg, Zn and Cu; the B is selected from one or two of Nb and V; the S is selected from one or more than one of Li, Na, K, Ti, Ag, B, Al, Ga and In, the x is not less than 0.00001 and is not more than 0.1; the m is not less than 0 and is more than 0.99; the n is not less than 0 and is not more than 1.99; the d is not less than 0 and is not more than 0.1; and the f is not less than 0 and is not more than 0.1. The blue-excited red fluorescent material not only has the properties of piezoelectricity, ferroelectricity and dielectricity, but also has the characteristic of blue-excited red emission, belongs to a photoelectric multifunctional material and can be widely applied to the fields of white light LED (Light Emitting Diode), optoelectronic integration, micro electromechanics, photoelectric sense, and the like.
Owner:TONGJI UNIV
Red fluorescent material and preparation method thereof
InactiveCN102154008AImprove stabilityNot easy to deliquescenceLuminescent compositionsSemiconductor devicesElectricityRare-earth element
The invention relates to a novel blue-light-excited red fluorescent material applied to a white light LED (Light Emitting Diode) technology and a preparation method thereof, belonging to the field of light-emitting materials. The blue-light-excited red fluorescent material has a general formula of Bi0.5+dNa0.5-yAy)1-xRxTiO3:fC, wherein R is selected from one or more than one of rare-earth elements Pr, Sm, Eu and Lu, A is selected from one or more than one of the same group of univalent elements K and Li, C is selected from one or more than one of Li, Na, K, Tl and Ag, 0.001<=x<=0.1, 0<=y<=0.5, 0<=d<=0.1, and 0<=f<=0.1. Blue-light-excited red fluorescent powder provided by the invention has good stability, is not easy to deliquesce, does not need cladding treatment, not only has piezoelectric, ferroelectric and dielectric properties, but also has the characteristic of exciting red light by blue light, belongs to a multifunctional photoelectric material, and can be widely applied to fields such as white light LEDs, photoelectricity integration, micro electro mechanical systems, photoelectricity sensors and the like.
Owner:TONGJI UNIV
Method for preparing lithium iron phosphate in ionic eutectic mixture
ActiveCN102491304ALow melting pointLow vapor pressurePhosphorus compoundsSteam pressureQuaternary ammonium cation
The invention discloses a method for preparing lithium iron phosphate serving as a lithium ion battery cathode material in an ionic eutectic mixture, which belongs to the technical field of preparation of electrochemical power supply materials. In the method, an ionic eutectic mixture obtained by compounding urea / carboxylic acid / alcohols with a quaternary ammonium salt and organic amine or an organic alkali serving as a regulating agent is taken as a reaction solvent as well as a template agent, and pure-phase lithium iron phosphate with high crystalizing performance is directly obtained with an ionic thermosynthesis method. Compared with synthesis of lithium iron phosphate by taking an imidazole ionic liquid as a solvent, the method has the advantages of higher easiness in designing and synthesizing quaternary ammonium cations with excellent template functions in the ionic eutectic mixture, effective adjustment and control of physical and chemical properties through a donor of hydrogen bonds, low-price and readily available raw materials, environmental compatibility, biodegradability, insensitivity to water and greater convenience for using; compared with synthesis of lithium iron phosphate with a high-temperature molten salt method, the method has the advantage of lower melting temperature of the ionic eutectic mixture; and compared with synthesis of lithium iron phosphate with a hydro-thermal method, the method has the advantage of higher safety due to extremely low steam pressure. The invention provides a novel method for preparing a lithium iron phosphate cathode material. The method has a wide application prospect in the field of lithium ion battery cathode materials.
Owner:NORTHEASTERN UNIV
Humic acid coated urea and production method thereof
InactiveCN104058898ARealize green environmental protectionIncrease profitAgriculture gas emission reductionFertilizer mixturesWater basedEmulsion
The invention provides multifunctional humic acid type coated urea and a production method thereof. The production method comprises the following step: by taking urea as an inner core, humic acid and derivatives thereof as a main coating material and carrier, and a water-based emulsion as viscose, adding corresponding functional components and various auxiliary materials, thus obtaining the multifunctional humic acid type coated urea. The multifunctional humic acid type coated urea has the functions of moisture retention, fertilizer maintenance, slow dissolution, slow release and the like, and the utilization ratio of the urea is improved more effectively; and since the coated urea is produced under the condition of normal temperature and pressure, the process is easy to operate and control and easy to realize continuous large-scale production.
Owner:YANSHAN UNIV
Salt of benzoylaminopyridine derivative and application thereof in medicines
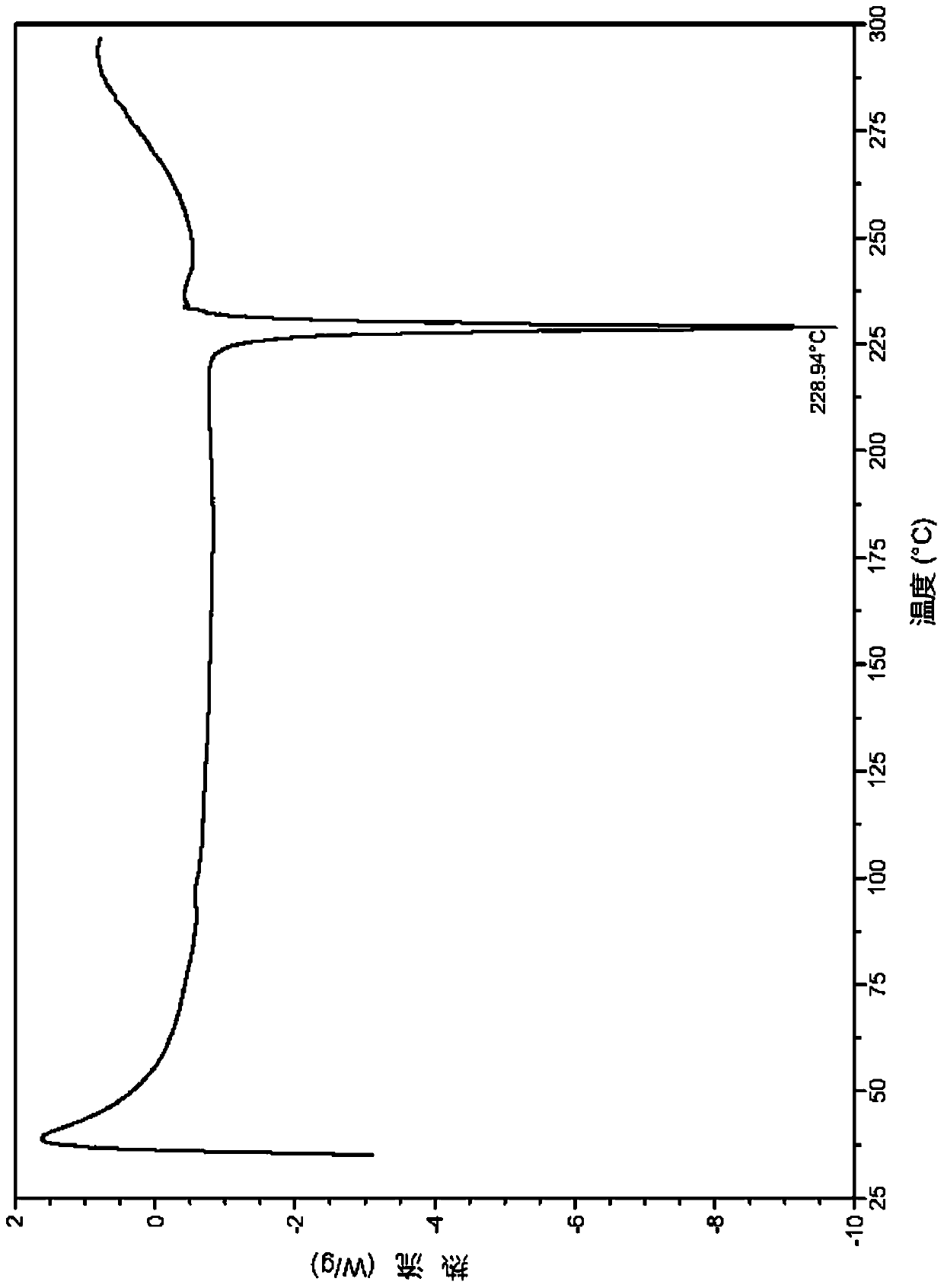
ActiveCN110577538ANot easy to deliquescenceConvenient for long-term storageOrganic active ingredientsNervous disorderMedicineCrystal
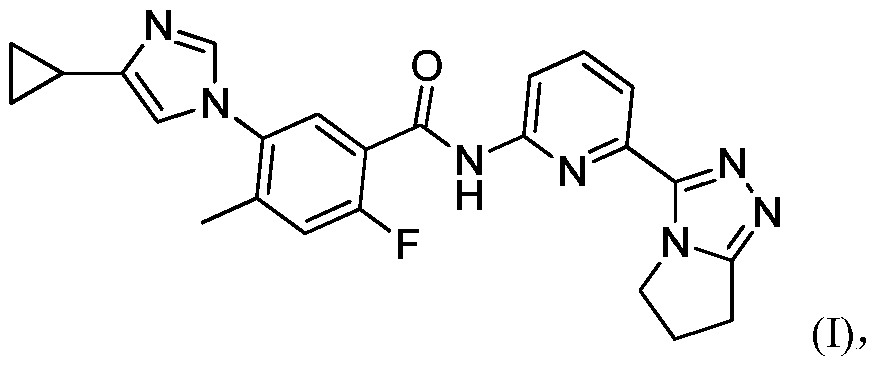
The invention relates to a salt of a benzoylaminopyridine derivative and application thereof in medicines, further relates to a pharmaceutical composition containing the salt or crystal form or a combination thereof, and application of the salt or crystal form or the pharmaceutical composition in preparing the medicines for preventing, treating or relieving diseases regulated by ASK1 of patients.
Owner:GUANGZHOU ANYANREN PHARMA TECH CO LTD
Salt of benzoylaminopyridine derivative and application thereof in medicines
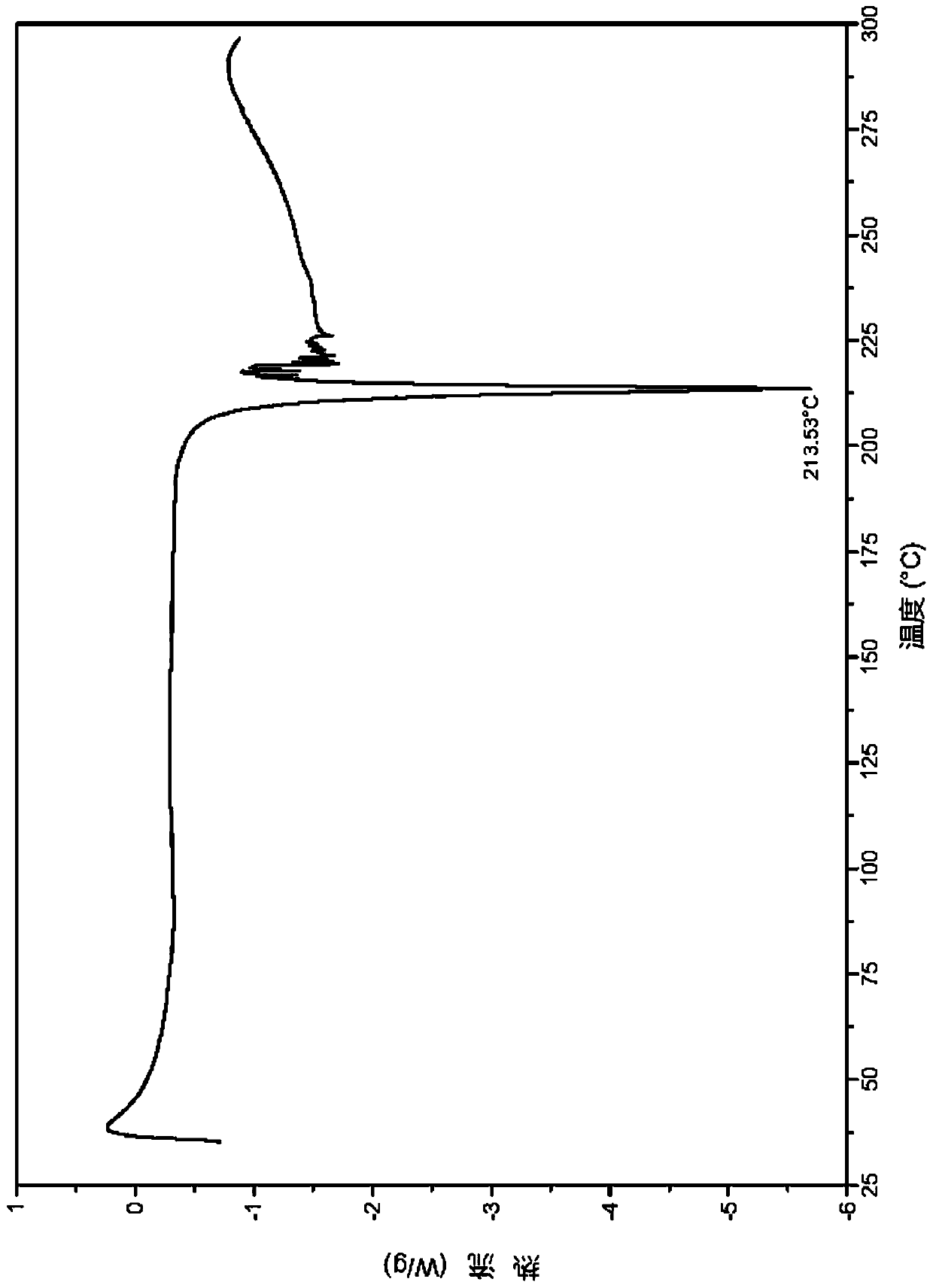
ActiveCN110577540ANot easy to deliquescenceConvenient for long-term storageOrganic active ingredientsNervous disorderMedicineCrystal
The invention relates to a salt of a benzoylaminopyridine derivative and application thereof in medicines, further relates to a pharmaceutical composition containing the salt or crystal form or a combination thereof, and application of the salt or crystal form or the pharmaceutical composition in preparing the medicines for preventing, treating or relieving diseases regulated by ASK1 of patients.
Owner:GUANGZHOU ANYANREN PHARMA TECH CO LTD
Borate birefringent crystal for ultraviolet band as well as growing method and purpose of same
InactiveCN103074684ACut wellGood groundingPolycrystalline material growthBy pulling from meltSpace groupFlux growth
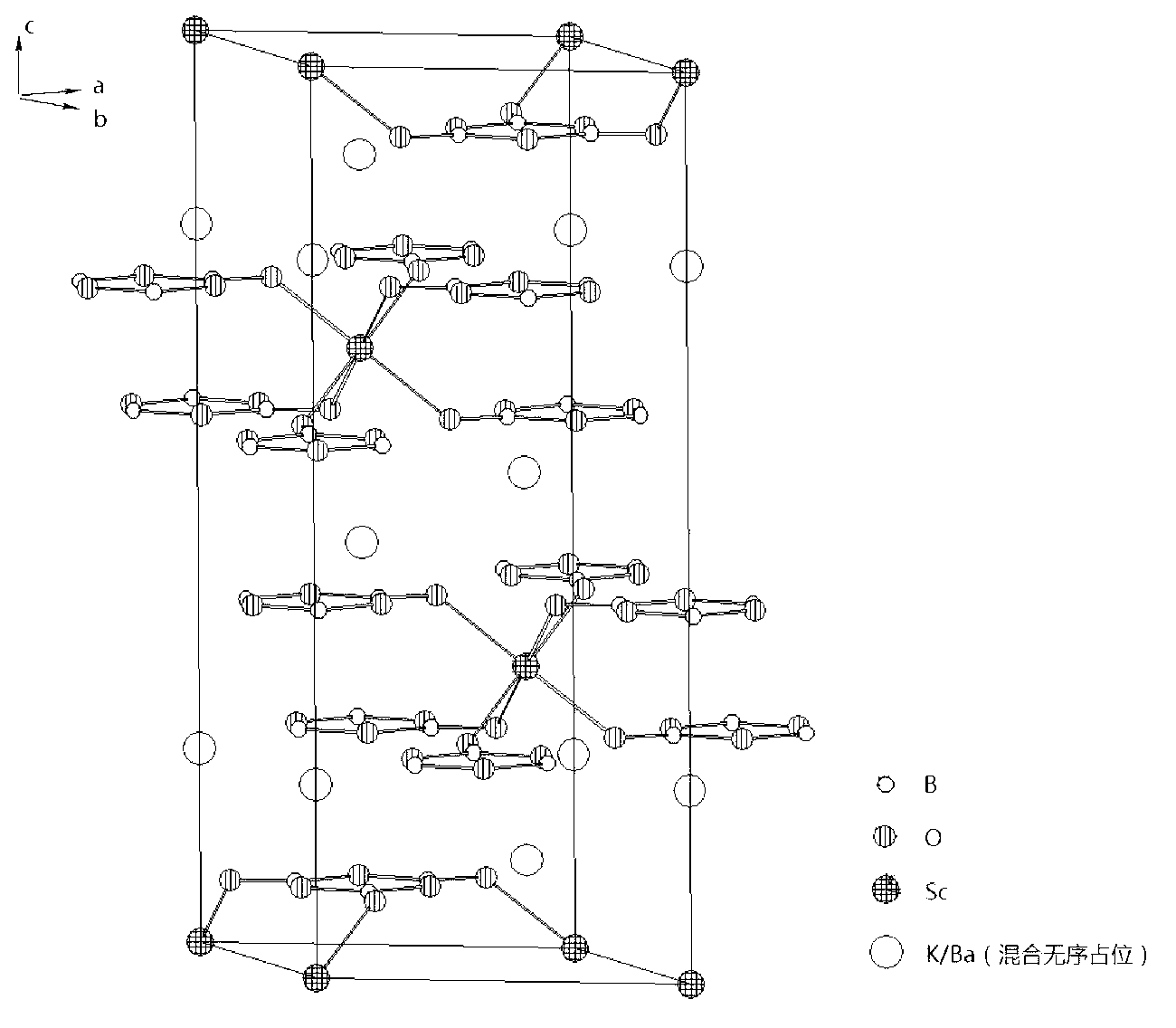
The invention relates to a borate birefringent crystal for an ultraviolet band. The chemical formula of the borate birefringent crystal is KBaSc(B3O6)2 and belongs to a trigonal system; an atom K and an atom Ba in a crystal structure are in disordered mixed occupation; a space group is R-3 and a cell parameter Z is 3; the transmission range is 184-3000 nm; a calculated value n0 of the refractive index at gamma of 589 nm is 1.6168, ne is 1.5079 and delta n is 0.1089; the crystal grows by using a self-melting and spontaneous crystallization method or a flux growth method; the crystal is easy to grow, cut, grind, polish and preserve, is stable in the air, is uneasy for deliquescence and is insoluble in water; the crystal can be used for making polarization beam splitters such as Glan prisms, Wollaston prisms, Rochon prisms or beam splitting polarizers; and the crystal is importantly applied to the optical and communication fields.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
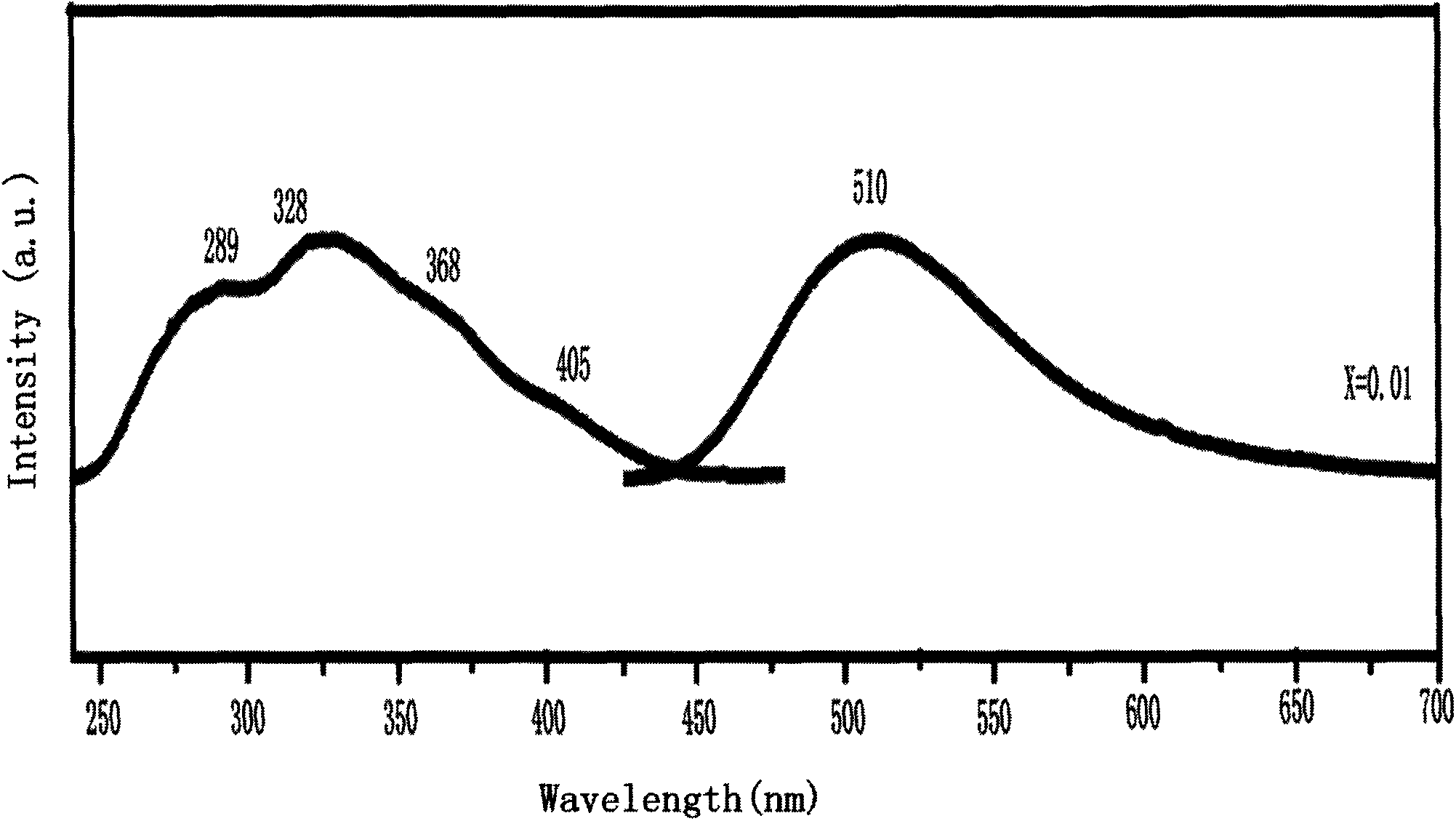
Europium ion activated silicon-based nitrogen oxide green fluorescent powder and preparation method thereof
InactiveCN103834391APromote reductionProcess stabilityLuminescent compositionsAlkaline earth metalGreen-light
The invention belongs to the technical field of a rare-earth luminescent material, and relates to europium ion activated silicon-based nitrogen oxide green fluorescent powder and a preparation method thereof. The europium ion activated silicon-based nitrogen oxide green fluorescent powder comprises chemical ingredients as shown in the following chemical formula: M3(1-x)Si2O4N2:3xEu<2+>, wherein the M element is an alkaline-earth metal element such as Ca, Mg, Sr, Ba and the like, x is not smaller than 0.002 and not larger than 0.01, and the Eu<2+> is doped rare-earth ion. The preparation method disclosed by the invention is used for synthesizing the green fluorescent powder M3(1-x)Si2O4N2:3xEu<2+> through a high-temperature solid-phase synthesis method. The green fluorescent powder emits intensive green light under excitation of near ultraviolet light; the absorbency is quite high within an excitation wavelength range of 270nm-450nm; the main emission peak is at about 510nm, and the green fluorescent powder has high luminous efficiency, high intensity and good thermal stability and can be used in white light LED (Light Emitting Diode) with high color rendering property; the preparation method of the green fluorescent powder is simple, gentle in reaction condition, easy to operate, energy-saving and time-saving, and has extremely good application prospect in solid illumination field.
Owner:CHINA JILIANG UNIV
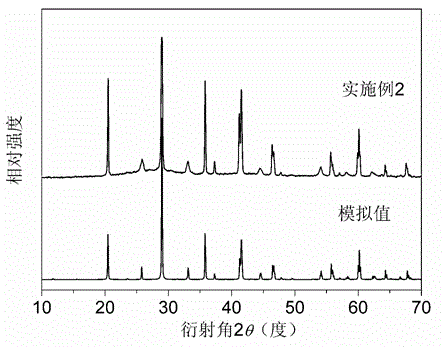
JAK inhibitor crystal forms, preparation methods and applications thereof
InactiveCN105061420AGood physical and chemical propertiesImprove stabilityOrganic active ingredientsAntipyreticPharmaceutical formulationChemical property
The present invention discloses four crystal forms of a JAK inhibitor N-(5-(4-(1,1-dioxothiomorpholinyl)methyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-yl)cyclopropanecarboxamide, and methods for preparing the four crystal forms, wherein the four crystal forms respectively are a crystal form H1, a crystal form H2, a crystal form H3 and a crystal form H4, the crystal form H1 has the characteristic absorption peaks when the diffraction angle 2[theta] is 8.3 DEG, 11.2 DEG, 16.0 DEG, 17.5 DEG, 18.5 DEG, 19.3 DEG, 19.7 DEG, 20.0 DEG, 20.7 DEG, 22.0 DEG and the like, the crystal form H2 has the characteristic absorption peaks when the diffraction angle 2[theta] is 9.3 DEG, 12.8 DEG, 14.0 DEG, 16.4 DEG, 18.7 DEG, 20.5 DEG, 23.5 DEG, 29.4 DEG, 33.1 DEG, 33.4 DEG and the like, the crystal form H3 has the characteristic absorption peaks when the diffraction angle 2[theta] is 9.6 DEG, 9.8 DEG, 10.7 DEG, 15.1 DEG, 15.3 DEG, 16.8 DEG, 16.9 DEG, 19.8 DEG, 20.0 DEG, 24.9 DEG and the like, and the crystal form H1 has the characteristic absorption peaks when the diffraction angle 2[theta] is 8.6 DEG C, 9.6 DEG, 10.5 DEG, 12.9 DEG, 15.1 DEG, 17.2 DEG, 18.9 DEG, 19.9 DEG, 20.7 DEG, 23.8 DEG and the like. According to the present invention, the four crystal forms have advantages of excellent physical and chemical properties, good stability, simple preparation operation and the like, are suitable for pharmaceutical formulation applications.
Owner:CHARM PHARMATECH NANJING
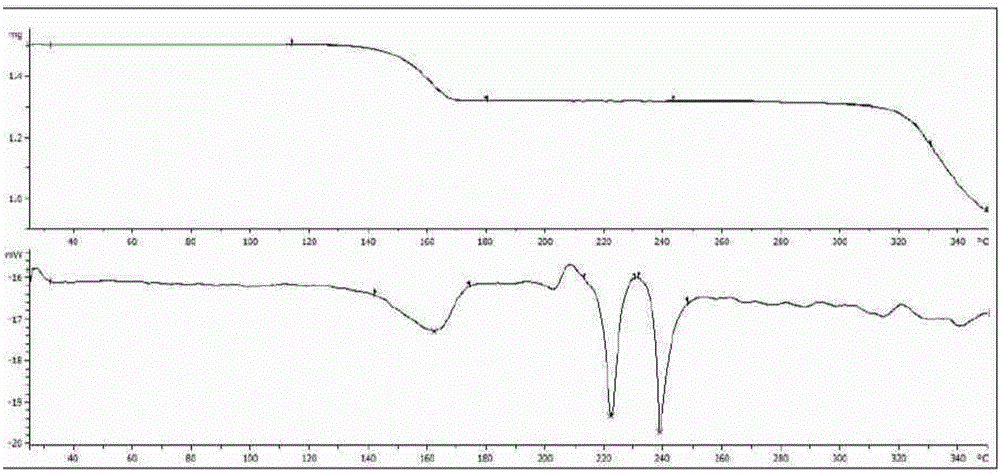
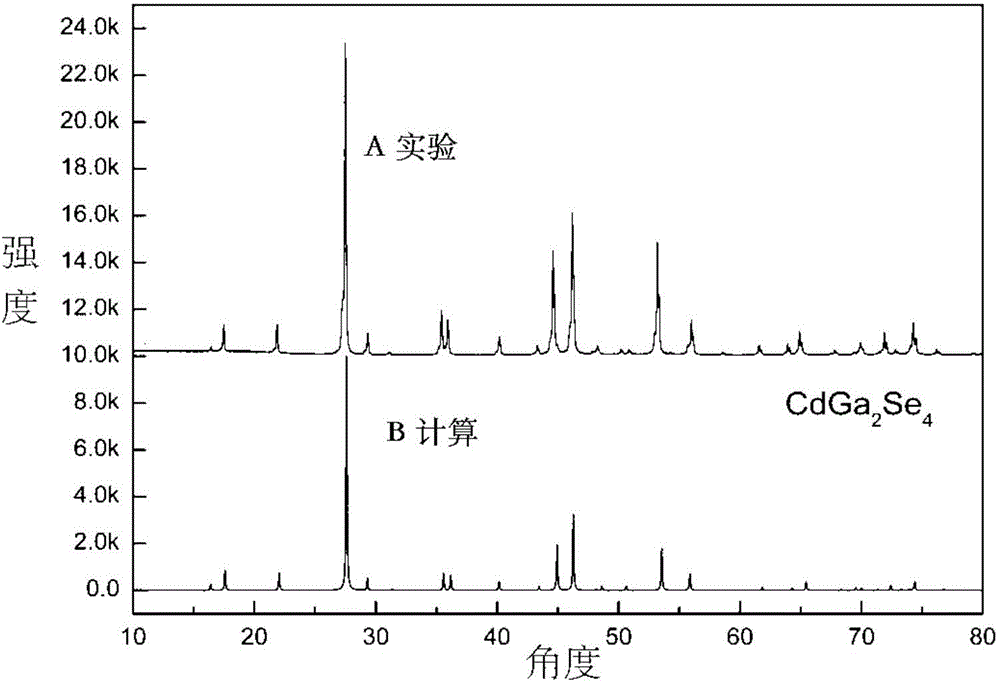
Long wave infrared nonlinear CdGa2Se4 crystal as well as growth method and use of crystal
ActiveCN104695022AImprove mechanical propertiesStable chemical propertiesPolycrystalline material growthFrom frozen solutionsSpontaneous nucleationLong wave infrared
The invention relates to a long wave infrared nonlinear CdGa2Se4 crystal as well as a growth method and the use of the crystal. The CdGa2Se4 polycrystal material is synthesized by virtue of a high-temperature solid-phase reaction; the long wave infrared nonlinear CdGa2Se4 crystal is grown by use of a spontaneous nucleation Bridgman-Stockbarger method or an oriented seed crystal assisted Bridgman-Stockbarger method; the frequency doubling effect of the obtained nonlinear optical CdGa2Se4 crystal powder is about three times of that of AgGaS2, and the transparent waveband of the infrared region of the CdGa2Se4 crystal powder is capable of reaching long wave infrared 21 microns; besides, the CdGa2Se4 crystal powder is good in mechanical properties, stable in chemical properties, not prone to deliquescence, and suitable for orientation, cutting and polishing. The long wave infrared nonlinear CdGa2Se4 crystal is applicable to manufacturing infrared nonlinear optical devices.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Preparation method of instant black tea
The invention discloses a preparation method of an instant black tea, which is characterized by comprising the following processing steps of: adopting water as a solvent to extract soluble substance in black tea leaves, and producing the extracting solution; concentrating the produced extracting solution for two times, and obtaining the concentrated solution; and refrigerating and subliming the concentrated solution, then drying, and obtaining the instant black tea. The instant tea obtained in the invention has the characteristics of strong tea aroma, good solubility, less impurities, difficulty in hygroscopy and limpid water solution, and has very good drinking taste.
Owner:罗朝雯
A potassium fluoborate compound, a potassium fluoborate non-linear optical crystal, a preparing method of the crystal and uses of the crystal
ActiveCN106948003APromote growthStable physical and chemical propertiesPolycrystalline material growthBy pulling from meltNonlinear optical crystalSpace group
A potassium fluoborate compound, a potassium fluoborate non-linear optical crystal, a preparing method of the crystal and uses of the crystal are provided. The chemical formula of the compound is KB4O6F, the molecular weight of the compound is 197.34, and the compound is prepared by a solid-phase synthetic method or a vacuum encapsulation method. The chemical formula of the crystal is KB4O6F, and the molecular weight of the crystal is 197.34. The crystal belongs to an orthorhombic system, and the space group of the crystal is Pna2<1>. According to cell parameters, a=7.4638 angstroms, b=11.2913 angstroms, c=6.5089 angstroms, alpha=beta=gamma=90 degrees, and the unit-cell volume is 548.54 angstrom<3>. The frequency-doubled effect of the crystal is 1.8 times of that of KH2PO4 (KDP), and the ultraviolet absorption edge is shorter than 190 nm. The crystal grows by adopting a melt method, a high-temperature melt method, a vacuum encapsulation method, a hydrothermal method or a room-temperature solution method. The crystal has good chemical stability, and can be applied as an ultraviolet and deep ultraviolet non-linear optical crystal in all-solid-state lasers.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Chlorine-free highly effective composite enhanced solid cement grinding aids and production method
The invention provides a chlorine-free high-efficiency composite reinforcing type solid cement grinding aid and production method, the grinding aid is composed of inorganic substrate material, organic material and water, strong antifreeze and antirust; the inorganic substrate material is composed of powder coal ash, sodium carbonate and sodium silicate, the mixing solution of any three or four among triethanolamine, glycol, propanediol, glycerolor paper pulp waste liquid is selected as the organic material, the inorganic strong antifreeze is composed of aluminum sulfate, calces or sodium hydrogen, sodium acetate or carbamide, and the antirust is sodium dihydrogen phosphate. The production method is the following: first the inorganic strong antifreeze is utilized to perform the complexation and modification of the organic material solution, and then the obtained mixing solution and the inorganic substrate material are furthermore compounded, reacted, blended and crushed. The invention has simple process, low cost, wide application range, good anti-freezing anti-rusting functions, and can improve later strength of cement obviously without disadvantage influence, and also obviously reduce the using amount of cement clinker by 20 percent, thereby having outstanding economical benefit.
Owner:DALIAN UNIV OF TECH
Rb3Al3B3O10F compound, Rb3Al3B3O10F nonlinear optical crystals as well as preparation method and use thereof
ActiveCN104556084ALarge nonlinear optical effectPhysicochemically stablePolycrystalline material growthFrom melt solutionsNonlinear optical crystalUv absorbance
The invention provides an Rb3Al3B3O10F compound, Rb3Al3B3O10F nonlinear optical crystals as well as a preparation method and use thereof and relates to the field of non-linear optical crystal materials. The frequency-doubling conversion efficiency of the Rb3Al3B3O10F nonlinear optical crystals at 1064nm is about 1.2 times of that of the KH2PO4 (KDP) crystal, ultraviolet absorption cutoff edges of the Rb3Al3B3O10F nonlinear optical crystals are shorter than 200nm and the Rb3Al3B3O10F nonlinear optical crystals do not absorb moisture; the large-size transparent Rb3Al3B3O10F nonlinear optical crystals can separately grow by a fluxing agent method and adopting RbF-B2O3 as a fluxing agent; and the Rb3Al3B3O10F crystals have stable physical and chemical properties and moderate hardness and are easy to cut, process, store and use and can be used for preparing non-linear optical devices so as to explore nonlinear optical applications of ultraviolet and deep-ultraviolet bands.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Stored grain pest insecticide and preparation method thereof
InactiveCN106490026AImprove insecticidal effectStrong fumigationBiocideDead animal preservationAdhesiveSitophilus
The invention discloses a stored grain pest insecticide. The insecticide comprises, by weight, 30-42 parts of vitex negundo linn, 30-42 parts of Chinese prickly ash, 20-30 parts of chenopodium ambrosioides, 22-30 parts of folium eucalypti, 1-4 parts of dispersing agent, 70-80 parts of carrier and 1-8 parts of adhesive. Various raw materials of the stored grain pest insecticide cooperate with each other, advantages are complementary, the corrected death rate of sitophilus zeamais, sitophilus zeamais motschusky, tribolium castaneum and bruchid is high, the insecticidal effect is remarkable, residues are not generated, and the insecticide is environmentally friendly, convenient to use and long in validity period.
Owner:ZHENGZHOU SIBIAN TECH CO LTD
Compound calcium fluoborate and calcium fluoborate nonlinear optical crystals and preparation method and application thereof
ActiveCN108588833APrevent burstLong growth cyclePolycrystalline material growthBy pulling from meltNonlinear optical crystalSpace group
The invention provides a compound calcium fluoborate and calcium fluoborate nonlinear optical crystals and a preparation method and application thereof. The chemical formula of the compound is CaB5O7F3 and the molecular weight is 263.13. The compound is prepared by a solid-phase synthesis or vacuum encapsulation method. The chemical formula of the crystals is CaB5O7F3; the molecular weight is 263.13; the crystals belong to the orthorhombic system; and the space group is Cmc21. The cell parameters are as follows: a=9.926(10) angstrom, b=8.400(8) angstrom, c=7. 966(8) angstrom, alpha = beta = gamma = 90 degrees, the unit cell volume is 664.2 (11) angstrom 3, the frequency-doubled effect of the crystals is about double the frequency-doubled effect of KH2PO4 (KDP), and the ultraviolet absorption edge is shorter than 190 nm. The crystals are grown by a melt method, a high temperature solution method, a vacuum encapsulation method, a hydrothermal method or a room-temperature solution method.The crystals have good chemical stability and can be used as ultraviolet and deep ultraviolet nonlinear optical crystals in an all-solid-state laser.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Multicompentnt granular urea and its production process
InactiveCN1357513ASuitable for growth needsOvercome the defect of single ingredientFertiliser formsUrea compound fertilisersEvaporationCa element
The multicomponent granular urea contains urea 60-80 wt%, ammonium sulfate 10-20 wt% and dolomite 10-20 wt% and has grain size of 2-4 mm. The production process includes evaporation of urea solution, preheating of ammonium sulfate and dolomite, mixing, granulation, cooling and packing. the multicomponnet granular urea can introduce S, Mg and Ca elements for the growth of crop while providing urea component, regulate soil nutrients and raise crop yield. It has delayed release function and high fertilizer effect.
Owner:黑龙江黑化集团有限公司
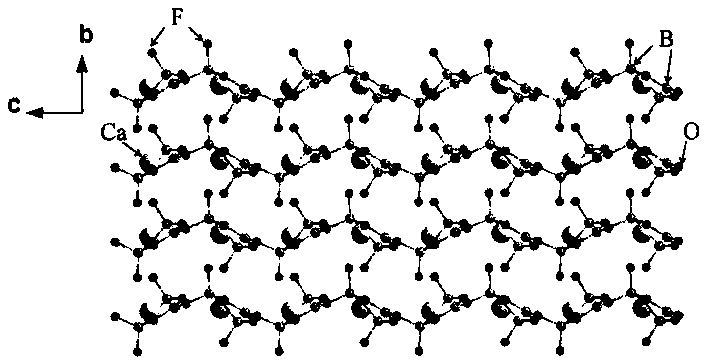
Method for hydrothermal growth of hydrated di-strontium-11-borate single crystal
InactiveCN101775653ANot easy to deliquescenceNot easy to dehydratePolycrystalline material growthFrom normal temperature solutionsDissolutionSingle crystal
The invention relates to a method for hydrothermal growth of a hydrated di-strontium-11-borate single crystal, which comprises selecting strontium salt and a boron-containing compound as raw materials, taking mineralizer solution or pure water as a dissolving medium, and preparing the hydrated di-strontium-11-borate single crystal through dissolution, hydration and crystallization of the strontium salt and the boron-containing compound in the hydrothermal medium. The specific steps are as follows: mixing the strontium salt and the boron-containing compound with the molar ratio of strontium and boron being 1:4-1:12, adding the mixture into a high-pressure reactor, and adding aqueous solution with 0-2.0mol / L of mineralizer with the filling degree of the high-pressure reactor being 50-80 percent; sealing the high-pressure reactor, placing the high-pressure reactor into the constant-temperature drying box with the temperature of 200-300 DEG C to keep the high-pressure reactor at the constant temperature for 3-14 days, switching off the power supply of the constant-temperature drying box, and lowering the temperature in the constant-temperature drying box to the room temperature; and taking out the product, washing the incompletely reacted raw materials with water, and drying the product, thereby obtaining the hydrated di-strontium-11-borate single crystal. The crystal has the advantage of powder frequency-doubling effect, and can not be easily deliquesced and dehydrated. Moreover, the operating conditions can be easily realized.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Mineral substance composite type cattle-and-sheep lick block and preparation method thereof
InactiveCN102524607AControl the amount of lickingQuality assuranceAnimal feeding stuffBrickManganese
The invention discloses a mineral substance composite type cattle-and-sheep lick block and a preparation method thereof. The mineral substance composite type cattle-and-sheep lick block per kilogram (kg) contains zinc sulfate 20-30g, copper sulfate 1.5-2.5g, ferrous sulfate 15-25g, manganese sulfate 1-2g, cobalt chloride 1-2g, potassium iodide 3-5g, sodium selenite 4-5g, magnesium sulfate 25-36g, sodium sulfate 25-37g, calcium sulfate 10-20g, calcium hydrophosphate 120-140g and sodium chloride 700-750g. The preparation method of the lick block ensures forming and proper hardness of the lick block, avoids the breaking, water loss and pulverization phenomena of the lick block due to deliquescence. The mineral substance composite type cattle-and-sheep lick block and the preparation method of the lick block has the advantages of being low cost and suitable for use in all regions.
Owner:SICHUAN ANIMAL SCI ACAD
Low-mercury catalyst for synthesizing vinyl chloride
InactiveCN102489306AReduce dosageGood synergyPreparation by halogen halide additionMetal/metal-oxides/metal-hydroxide catalystsRare earthChloride
The invention relates to a low-mercury catalyst for synthesizing vinyl chloride. The catalyst is characterized by comprising the following components, by weight: 2.5-3.5% of HgCl2; 0.1-12.5% of BiCl3; 0.15-15% of ZnCl2; 0.1-7.00% of TiCl4; 2.75-3.25% of CuCl2; 0.3-8.3% of VCl3; 0.3-8.3% of rare earth chloride; 3-8.5% of BaCl2; and 62.5-75% of C. In the invention, BiCl3, ZnCl2, TiCl4, CuCl2, VCl3, as well as BaCl2 that are cheap and has catalytic performance and rare earth chloride are employed to substitute part of HgCl2 for preparing the low-mercury catalyst, the HgCl2 content of which can be reduced to less than 3.5% while the catalytic performance is fully guaranteed. Thus, not only is the energy consumption reduced, but the product cost of the catalyst is also lowered. Prepared by a step impregnation method, the low-mercury catalyst in the invention is characterized by simple process, effectiveness, energy saving, environmental protection, and convenient operation, and simultaneously can overcome problems existing in prior art, thus being suitable for the second-stage process of vinyl chloride synthesis.
Owner:SHAANXI BEIYUAN CHEM GROUP +1
Slow-release long-acting solid toilet cleaner and preparation method thereof
InactiveCN108300598AReduce the amount addedRelease stabilityInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsRetention periodAlpha-olefin
The invention discloses a slow-release long-acting solid toilet cleaner. The slow-release long-acting solid toilet cleaner is prepared from the following components in parts by weight: 50 to 60 partsof sodium sulfate, 5 to 10 parts of food-grade carboxymethyl cellulose, 3 to 6 parts of hydroxyethyl cellulose ether, 3 to 6 parts of PVA (Polyvinyl Acetate), 5 to 10 parts of baking soda, 5 to 10 parts of sodium dodecylbenzene sulfonate, 3 to 5 parts of a surfactant AOS (Alpha Olefin Sulfonate), 3 to 5 parts of a pigment, 0.8 to 1 part of 5-chloro-2-methyl-4-isothiazolin-3-one, 0.05 to 0.1 part of essence, 0.05 to 0.1 part of terpineol and 9 to 10 parts of water. The slow-release long-acting solid toilet cleaner disclosed by the invention does not contain an acidic component, and the adding amount of the water is reduced; the carboxymethyl cellulose, the hydroxyethyl cellulose ether and food-grade carboxymethyl cellulose ether are added as stable components and slow-release components; aformula is scientific, and toilet cleaning active components are slowly released, the utilization time can be increased ,and the retention period of a product is long. The invention further disclosesthe slow-release long-acting solid toilet cleaner; technological steps are simple, special requirements on equipment are avoided, and the operability is high.
Owner:SHANGHAI MILE CHEM NEW MATERIALS CO LTD
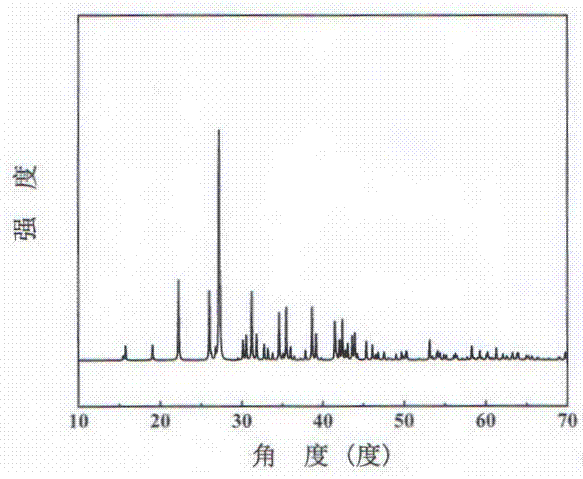
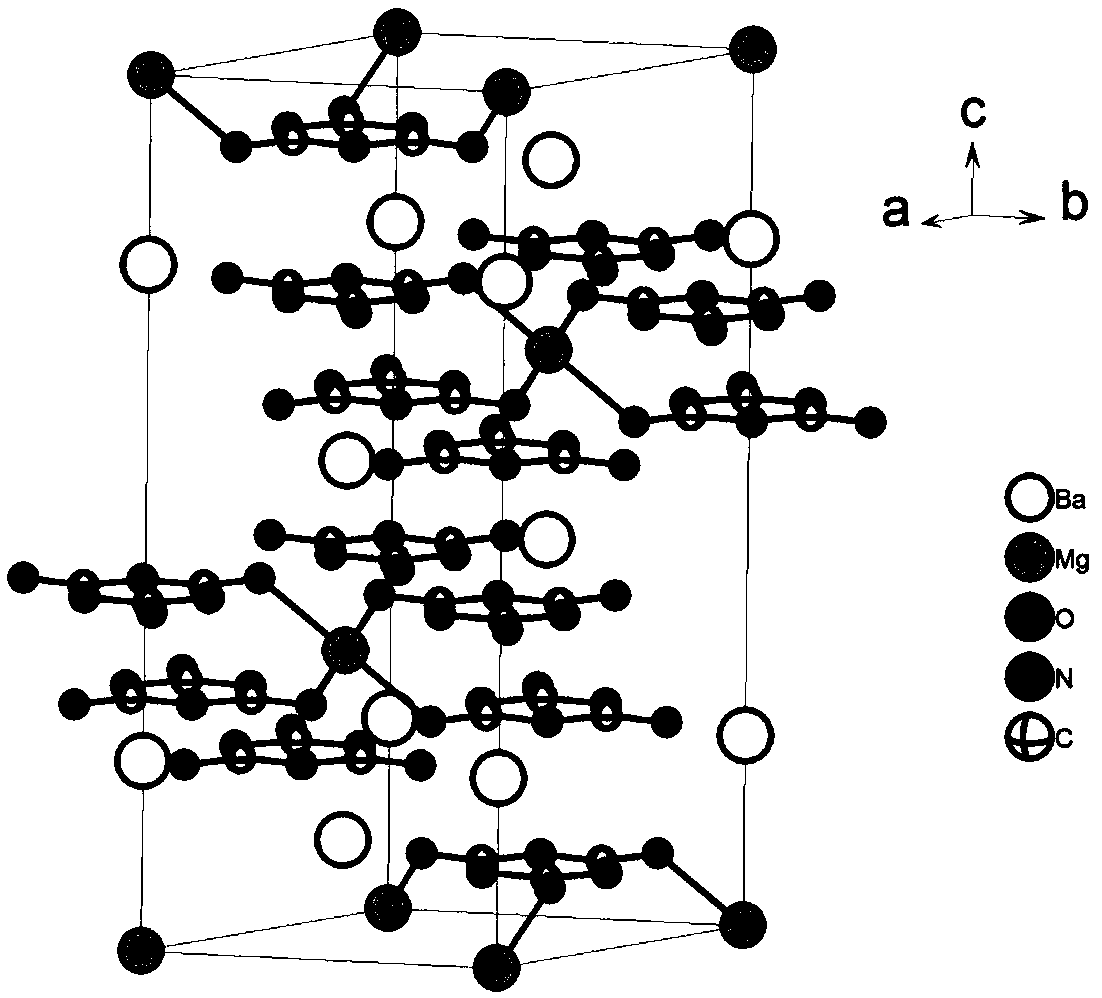
Barium magnesium cyanurate birefringent crystals for ultraviolet visible wavebands and preparation method and application of crystals
InactiveCN109137071APromote growthEasy to processPolycrystalline material growthFrom frozen solutionsSpace groupBeam splitting
The invention discloses barium magnesium cyanurate birefringent crystals for ultraviolet visible wavebands and a preparation method and application of the crystals. The chemical formula is Ba2Mg(C3N3O3)2 and belongs to a trigonal system, and the space group and unit-cell parameters are shown in the descriptions. The crystals grow by means of a high-temperature melt spontaneous crystallization method or a Bridgman-Stockbarger method. The barium magnesium cyanurate birefringent crystals obtained by means of the method are uniaxial negative crystals, the transmission range is 230-3,000 nm, and the calculation values of the refractive indexes at the place where lambda is equal to 800 nm are as follow:no is equal to 2.00, ne is equal to 1.649, and deltan is equal to 0.351. The crystals easily grow and are easy to cut, grind, polish and store, stable in air, uneasy to deliquesce and insoluble in water; the crystals can be used for manufacturing Glan prisms, Wollaston prisms, Rochon prisms orbeam splitting polarization device or other polarization beam splitting prisms and have important application in the field of optics and communications.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
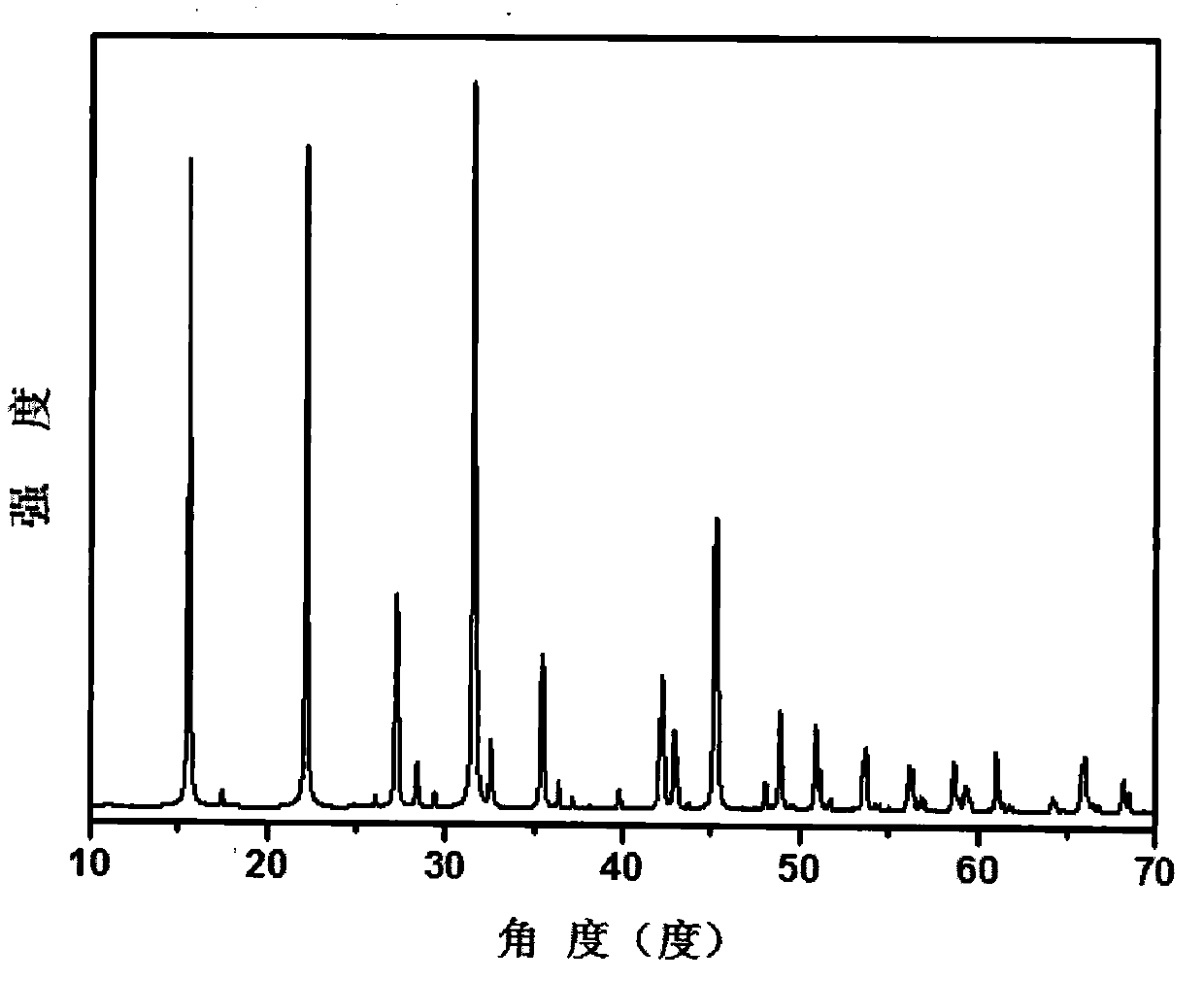
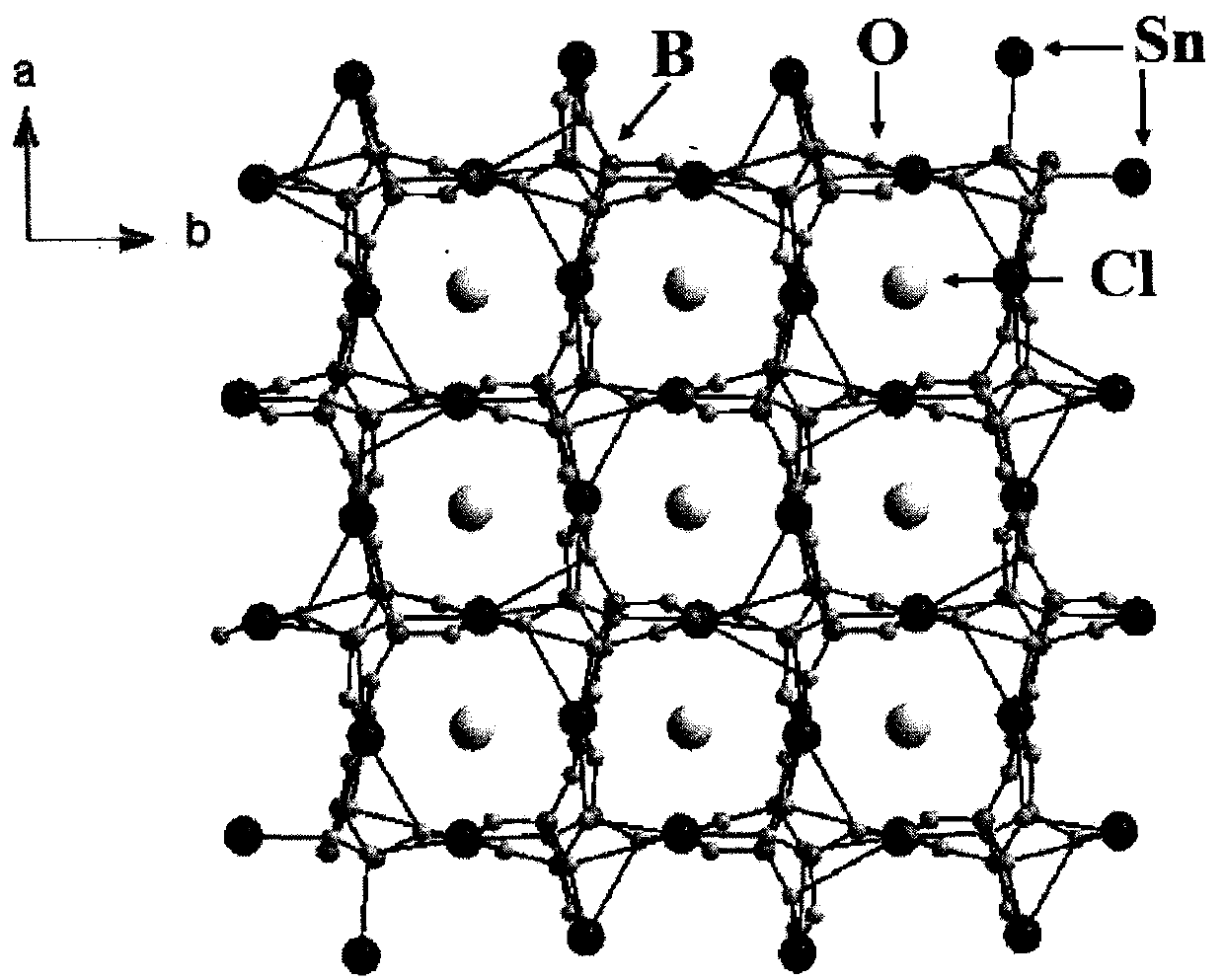
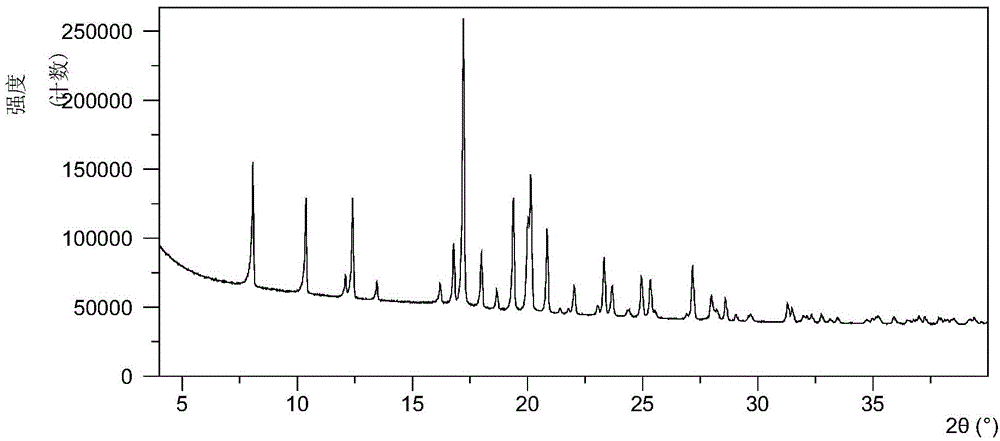
Compound tin-boron-oxygen-chlorine and tin-boron-oxygen-chlorine non-linear optical crystal as well as preparation method and application
InactiveCN109680332ALong growth cycleFast growthPolycrystalline material growthBy pulling from meltNonlinear optical crystalSpace group
The invention provides a compound tin-boron-oxygen-chlorine and a tin-boron-oxygen-chlorine non-linear optical crystal as well as a preparation method and application. The compound has a chemical formula Sn2B5O9Cl, has a molecular weight of 470.70, and is prepared by using a solid-phase synthesis method or a vacuum packaging method. The compound of the crystal has a chemical formula Sn2B5O9Cl, hasa molecular weight of 470.70, belongs to an orthorhombic crystal system, has a space group of Pnn2, has cell parameters that a=11.281(3) angstrom, b=11.331(3) angstrom, c=6.5573(19) angstrom, alpha=90 degrees, beta=90 degrees and gamma=90 degrees, and has a unit cell volume of 838.2(4) angstrom<3>; a frequency-doubled effect of the crystal is about 0.5 time of that of KH2PO4(KDP); an ultravioletcut-off edge of the crystal is less than 350nm. The crystal is grown by using a melting method, a high-temperature liquid melting method, a vacuum packaging method, a hydrothermal method or a room-temperature solution method, the crystal is good in chemical stability and can be applied to all-solid-state laser emitters as a short wavelength non-linear optical crystal.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Crystal form A of sofosbuvir and preparation method thereof
InactiveCN104974205AImprove solubilityImprove efficacyOrganic active ingredientsSugar derivativesSolubilityHigh humidity
The invention provides a crystal form A of sofosbuvir and a preparation method thereof. The crystal form A is characterized in that an X-ray powder diffraction spectrum has characteristic peaks at the 2[theta] value being 12.4 + / - 0.2 degrees, 19.4 + / - 0.2 degrees, 27.1 + / - 0.2 degrees, 13.5 + / - 0.2 degrees, 25.5 + / - 0.2 degrees and 16.8 + / - 0.2 degrees. The crystal form A is higher in solubility in a bio-medium, is beneficial to drug efficiency increasing, can reduce the carrying amount of the drug, is low in hygroscopicity and is not liable to deliquesce due to high humidity, so that the drug can be stored for a long time conveniently.
Owner:CRYSTAL PHARMATECH CO LTD
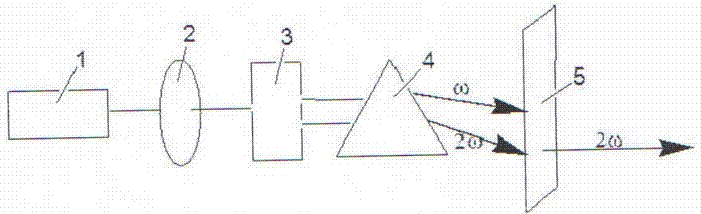
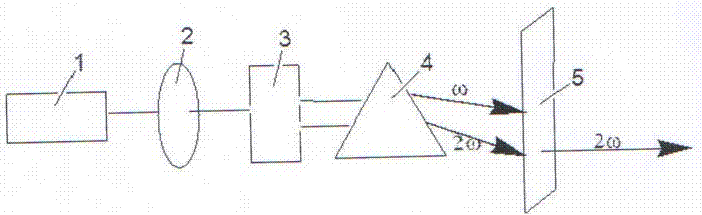
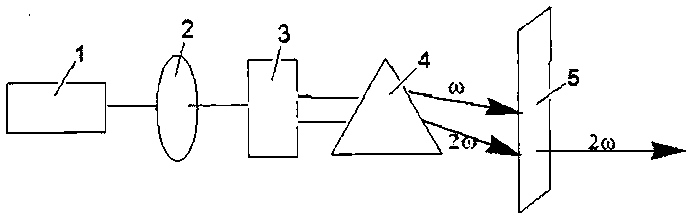
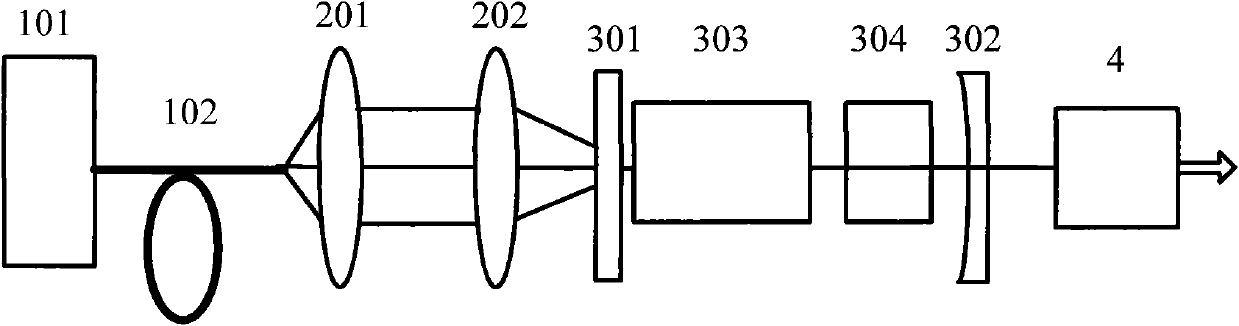
All solid-state 455nm pulsed laser based on neodymium-doped fluorinated lutetium lithium crystal
InactiveCN101950917AReduce volumeCompact structureOptical resonator shape and constructionActive medium materialFiberAll solid state
The invention relates to an all solid-state 455nm pulsed laser based on a neodymium-doped fluorinated lutetium lithium crystal, which is suitable for underwater laser communications. The all solid-state 455 nm pulsed laser is characterized by comprising a laser diode output with tail fibers, and a pump optical coupling system, a laser cavity and a multiple frequency crystal are sequentially arranged along the advanced direction of the laser diode outputting a laser, wherein the laser cavity comprises an Nd: LiLuF crystal and an electrooptic Q-crystal. The invention has the characteristics of compact structure, small size, long life and stable working.
Owner:SHANGHAI INST OF OPTICS & FINE MECHANICS CHINESE ACAD OF SCI
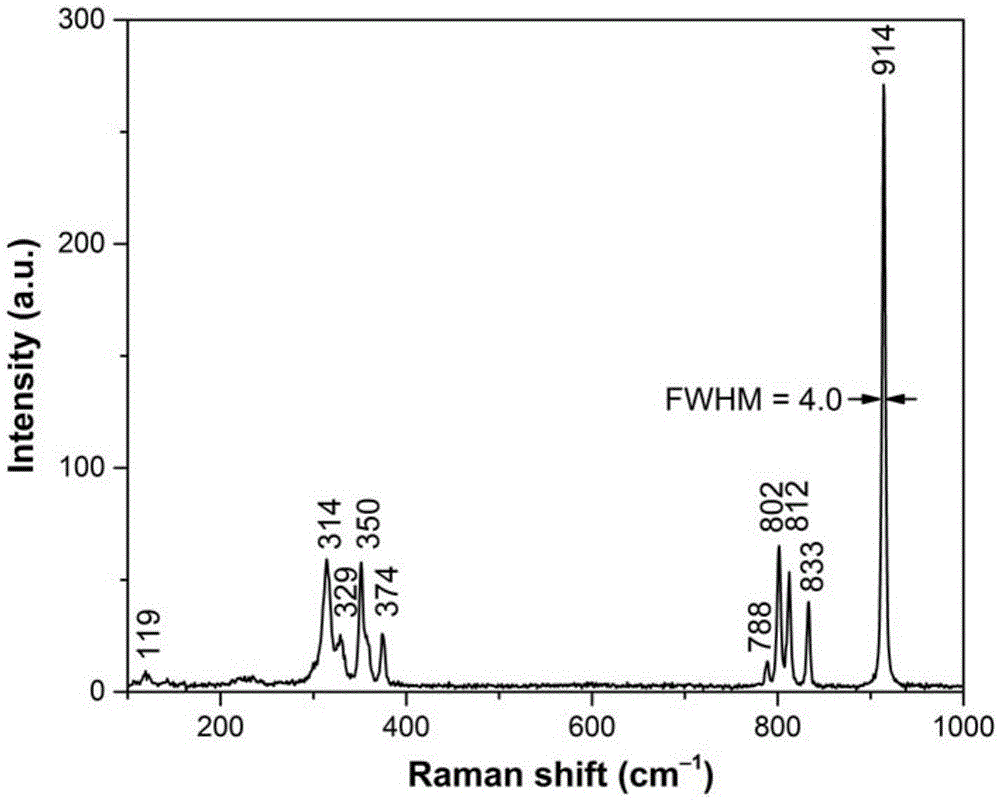
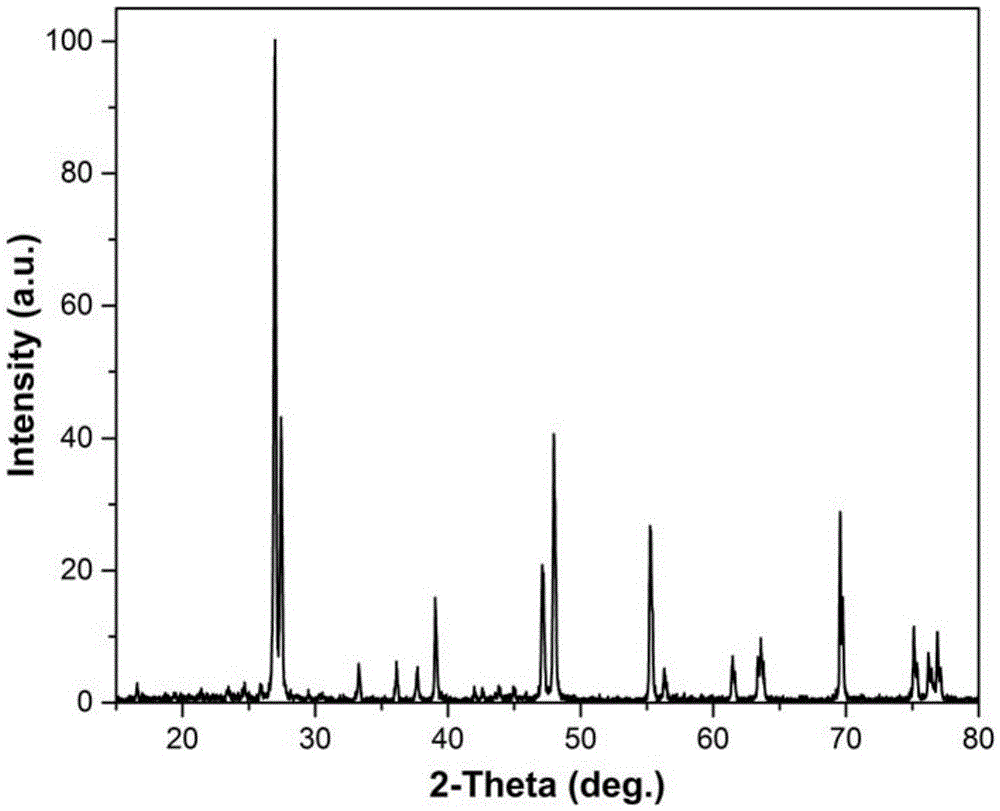
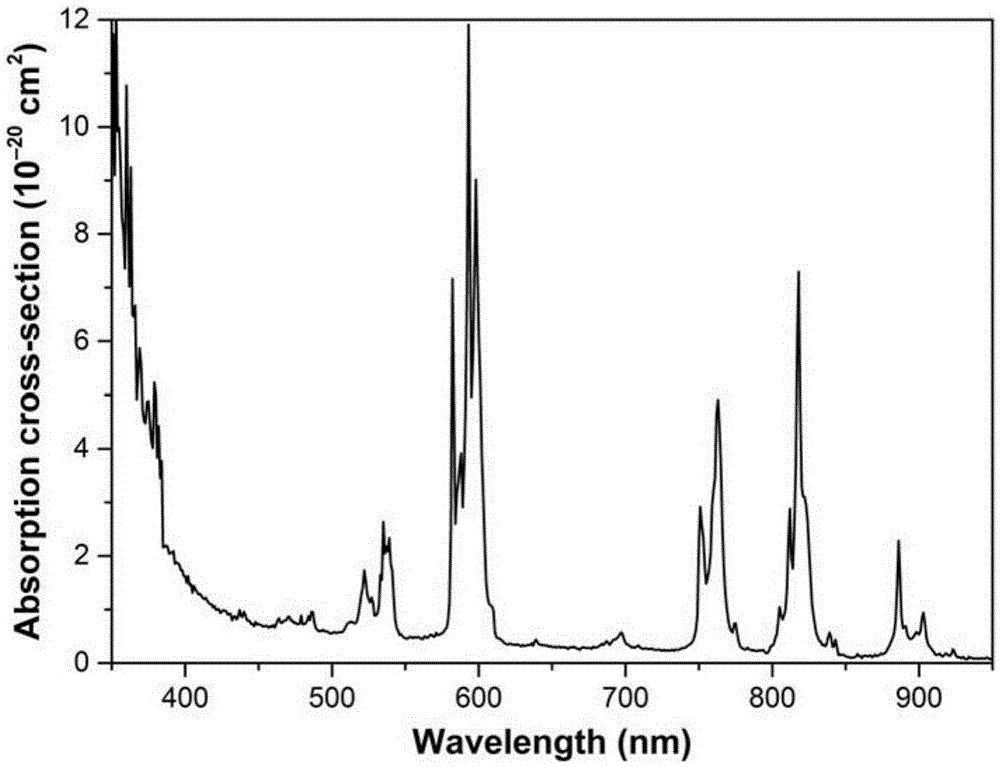
Multifunctional caesium lanthanum molybdate crystal as well as preparation method and application thereof
ActiveCN105463570ANarrow line widthGuaranteed OrientationPolycrystalline material growthLaser using scattering effectsLanthanumRaman laser
The invention discloses a multifunctional caesium lanthanum molybdate crystal as well as a preparation method and an application thereof and belongs to the technical field of artificial crystals. The chemical formula of the caesium lanthanum molybdate crystal is CsLa(1-x)Ndx(MoO4)2, wherein the doping concentration of Nd is larger than or equal to 0 and smaller than or equal to 0.15. Cs2MoO4 is taken as a flux, the mole ratio of the flux to CsLa(1-x)Ndx(MoO4)2 is 0.2-3, and the high-quality CsLa(1-x)Ndx(MoO4)2 crystal with a larger size is obtained through growing with a flux method at the cooling speed of 0.5-3 DEG C per day and at the rotating speed of 5-30 revolutions per minute. The caesium lanthanum molybdate crystal can be taken as a laser crystal to generate laser, can also be taken as a Raman crystal to perform Raman frequency conversion on the laser and can further be taken as a self-Raman laser crystal with a composite function for manufacturing of integrated, small and multi-wavelength lasers. A solid laser prepared from the crystal can be used for multiple fields such as military, medical treatment, remote sensing, ocean exploration and the like.
Owner:HUAINAN NORMAL UNIV
Transmission-type online detection device for coal characteristic indexes
InactiveCN102841106AIncrease profitImprove detection efficiencyMaterial analysis by transmitting radiationElectricityTransmission belt
The invention discloses a transmission-type online detection device for coal characteristic indexes. Neutrons generated by an electrically controllable deuterium-tritium micro-accelerator with an electrically controllable range of 14-17 MeV are irradiated on coal flow passing through a transmission belt and nuclearly reacted with main nuclides in the coal flow, so as to generate characteristic gamma rays; the characteristic gamma rays are unique, like human fingerprints and are commonly called nuclear fingerprints; the nuclear information is collected by utilizing a special sensor; all the main nuclides in coal can be detected on line by utilizing modern mathematical analysis technology according to the properties of the characteristic gamma rays; and moisture content, ash content and a heat value in the coal are analyzed in combination with a neutron moisture detection technology. The transmission-type online detection device has the characteristics as follows: elements and industrial characteristic indexes in all coal flow can be detected on line in real time without influence of coal types and granule sizes.
Owner:南京威测环保科技有限公司
Europium ion activated silicon phosphate green fluorescent powder and its preparation method
InactiveCN103952151APromote reductionProcess stabilityEnergy efficient lightingLuminescent compositionsPhosphateSemiconductor chip
The invention belongs to the technical field of rare earth luminescent materials, and relates to a europium ion activated silicon phosphate green fluorescent powder and its preparation method. The green fluorescent powder has the following chemical components shown in the following chemical formula: M5-x (PO4) 2SiO4:xEu <2 +>, where M element is one or more from Ca, Mg, Sr or Ba, 0.001 <=x <= 0.1, M5-x (PO4) 2SiO4 is a matrix, Eu <2 +>is a doped rare earth ion. The silicon phosphate green fluorescent powder M5-x (PO4) 2SiO4:xEu <2 +> is synthesized by a high temperature solid phase synthesis method, under excitation of near UV (ultra violet), the silicon phosphate green fluorescent powder has a strong absorption in the range of excitation wavelength of 220-450nm, has a wider emission peak in the range of 430-700nm, is suitable for excitation of near UV of 350-430nm, and is very consistent in emission wavelength with a near ultraviolet semiconductor chip; the silicon phosphate green fluorescent powder has the advantages of high luminous efficiency, high strength and stable chemical property, and is in a green light wave band; the preparation method of europium ion activated silicon phosphate is simple and easy to operate and can be used in industrial production, and the europium ion activated silicon phosphate green fluorescent powder can be used in high color rendering white-light LED, and ahs excellent application prospects in solid state lighting field.
Owner:CHINA JILIANG UNIV
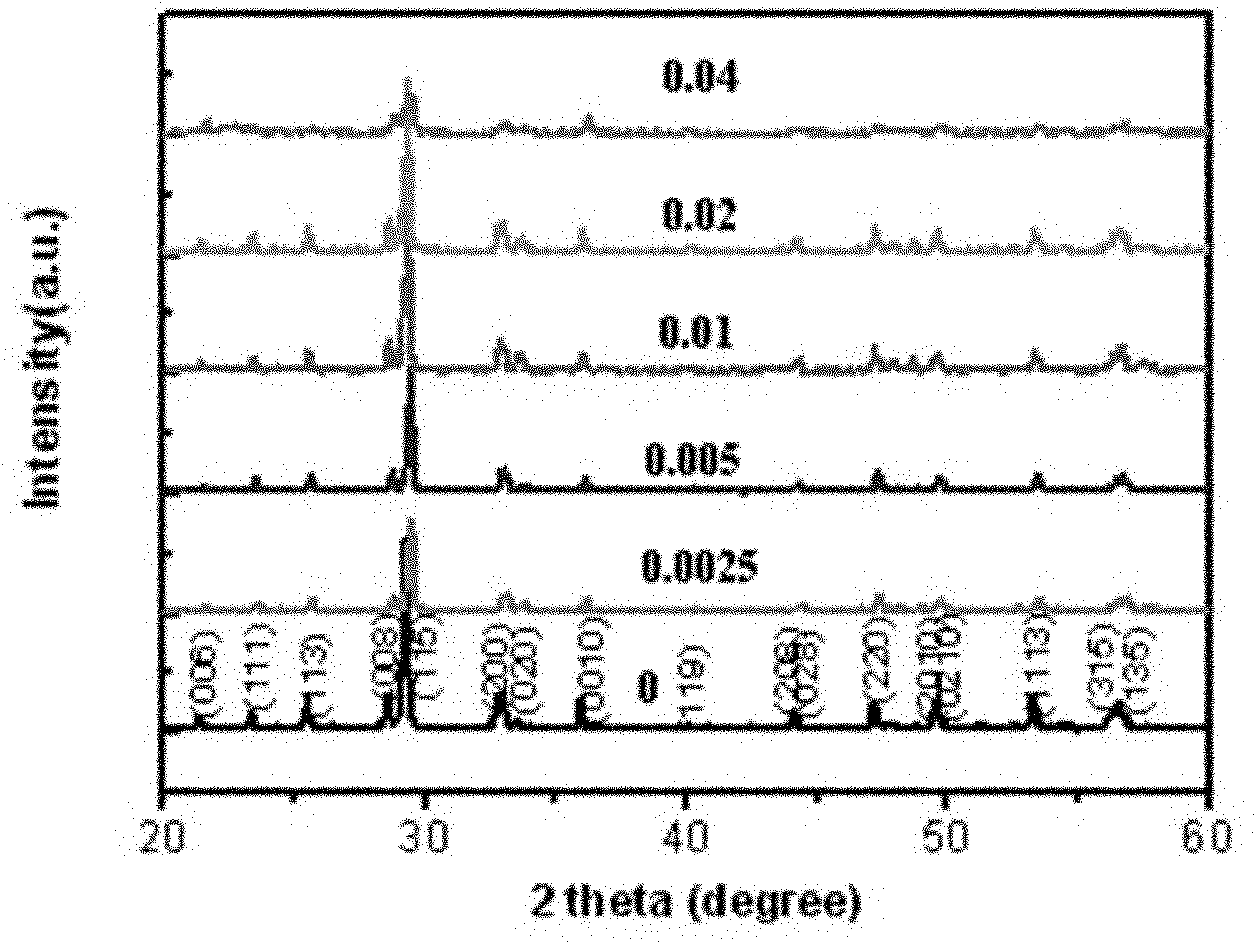
Photoelectric functional crystal M3RE(PO4)3 and preparation method thereof
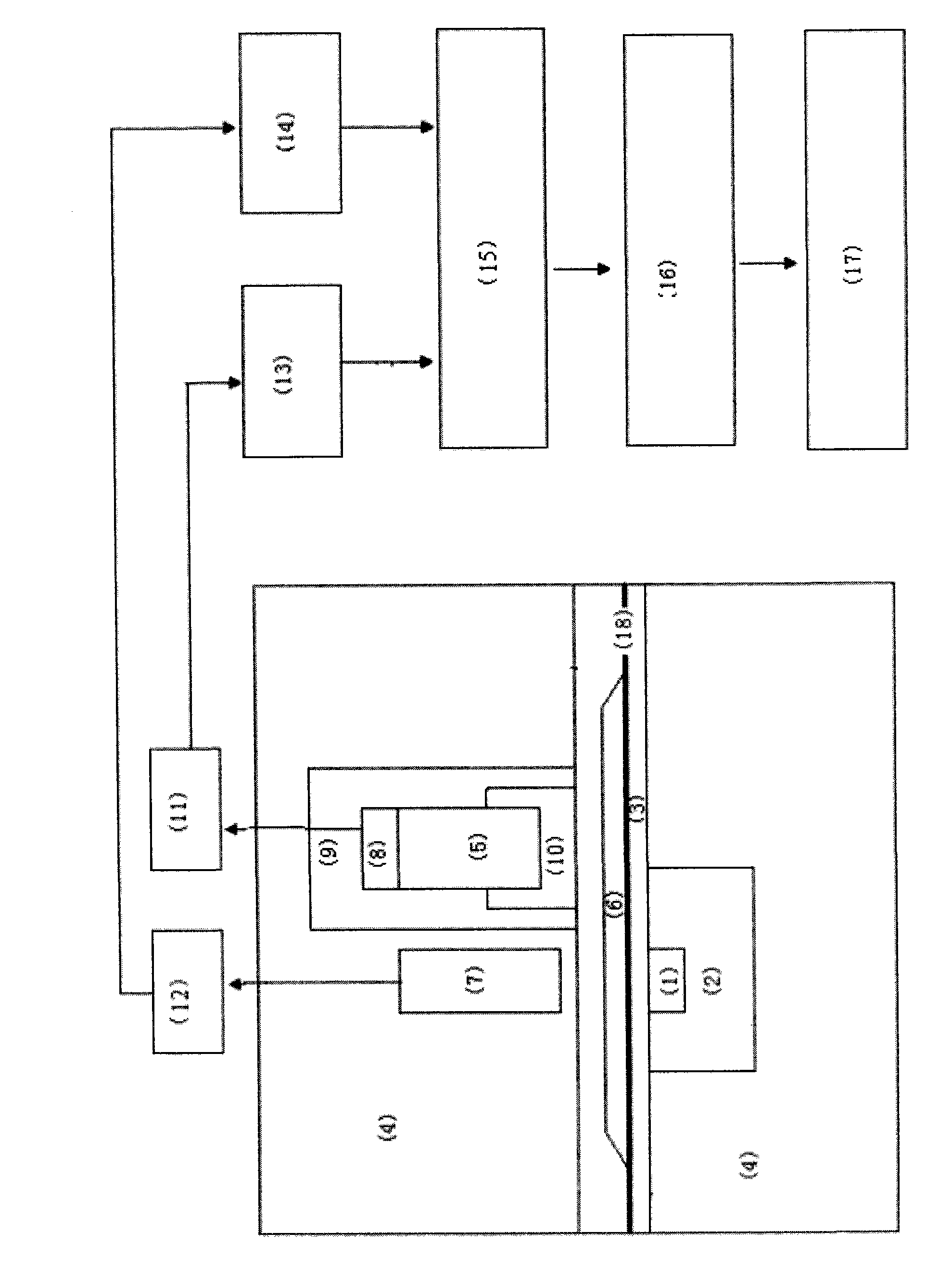
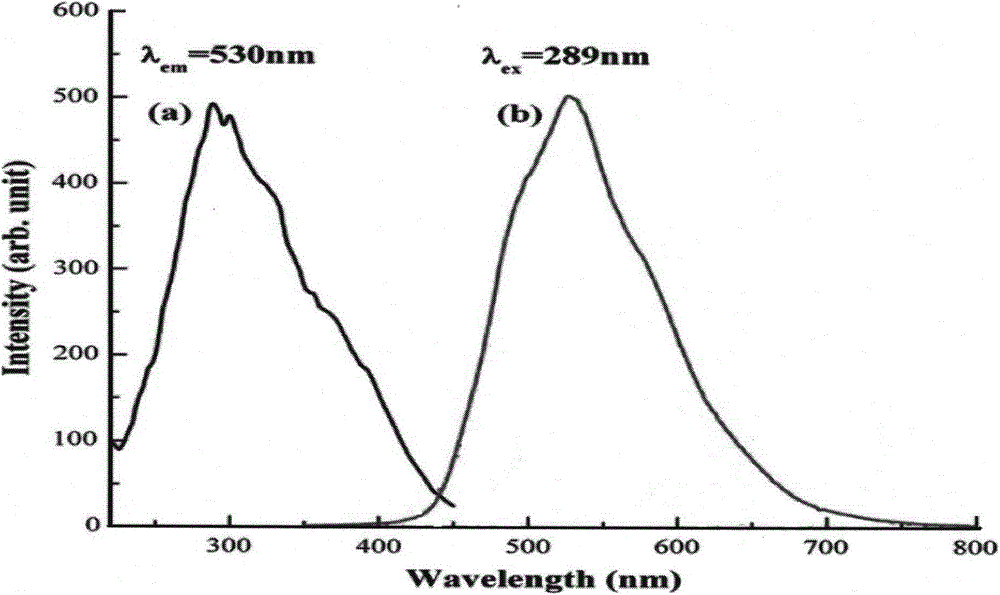
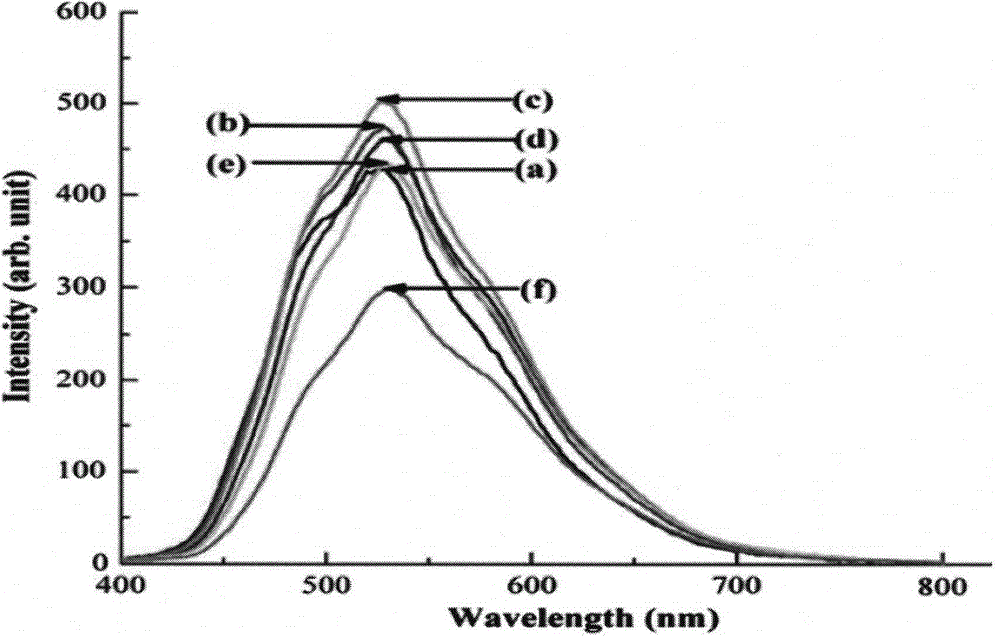
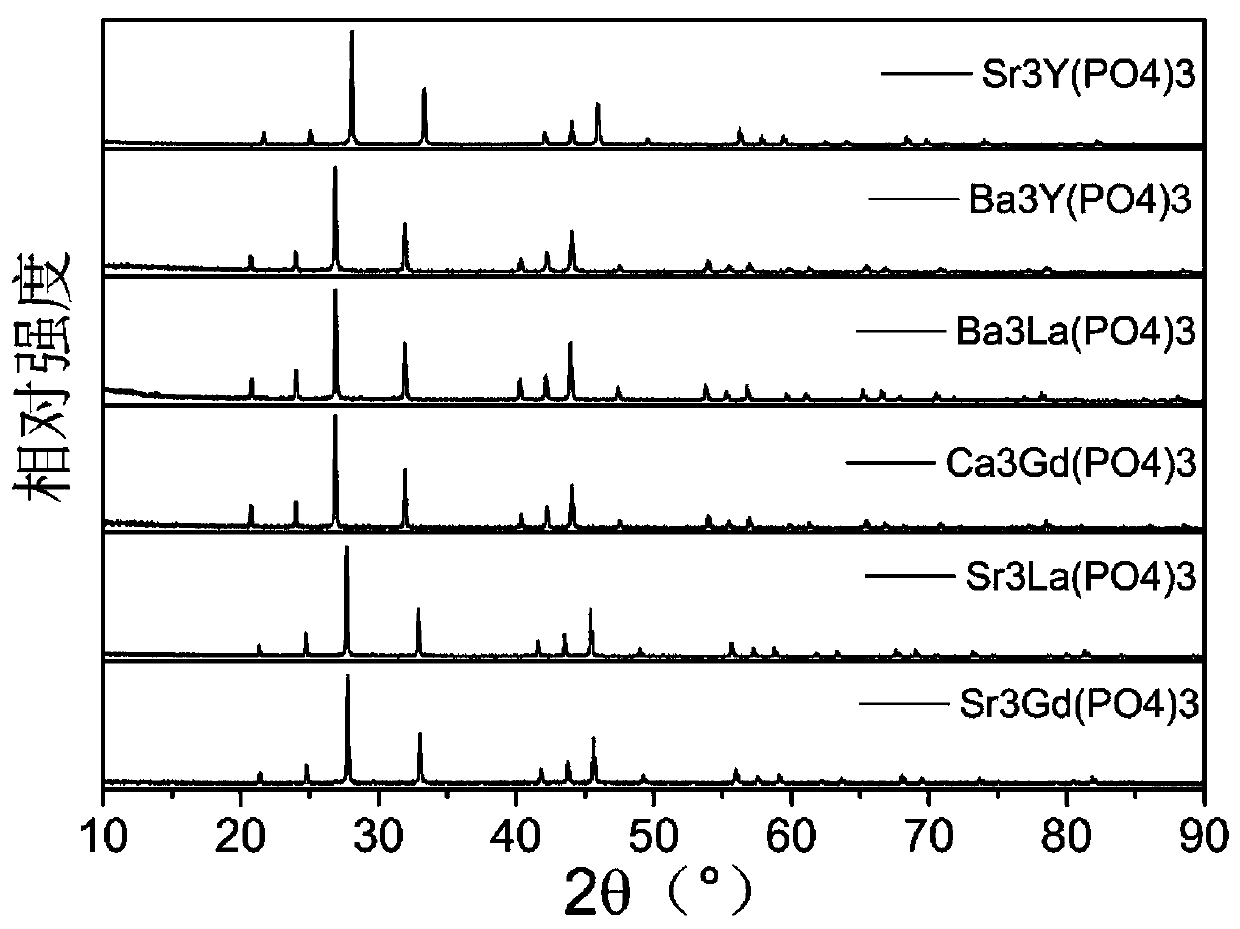
ActiveCN110067024ACtiveWith characteristicsPolycrystalline material growthBy pulling from meltCubic crystal systemFrequency conversion
The invention relates to a photoelectric functional crystal M3RE(PO4)3 and a preparation method thereof. The M3RE (PO4) 3 crystal is of a non-centrosymmetric structure, and belongs to a cubic system-43m point group, wherein M=Ba or Ca or Sr, RE=Y or La or Gd. The M3RE(PO4)3 crystal growth method comprises the steps of (1) polycrystal material synthesis, wherein stoichiometric MCO3, RE2O3 and a phosphorus compound are adopted, the phosphorus compound is excessive, and raw materials are sintered twice to obtain a M3RE(PO4)3 polycrystal material; (2) polycrystal material melting; (3) Czochralskicrystal growth. The prepared M3RE(PO4)3 crystal material is a high quality single crystal, not only are high optical transmittance and wide absorption edge achieved, but also no phase change exists from the room temperature to a melting point, deliquescence does not exist, and the piezoelectric activity and non-linear frequency conversion characteristics are achieved.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com