Salt of benzoylaminopyridine derivative and application thereof in medicines
A drug and pharmaceutical technology, applied in the field of L-tartrate pharmaceutical composition and L-tartrate crystal form, can solve the problems of undisclosed compound crystal structure, no disclosure, etc., and achieve the effect of convenient long-term storage and placement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1 L-tartrate crystal form I
[0083] 1. Preparation of L-tartrate crystal form I
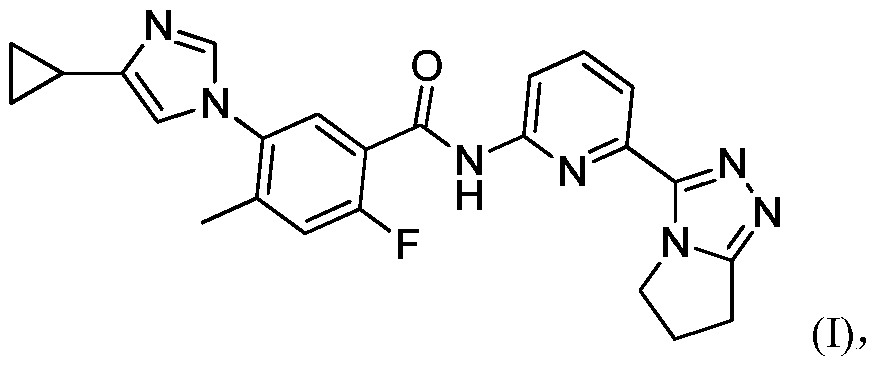
[0084] Compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2 ,4] Triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (50.3mg, 0.113mmol) was added to tetrahydrofuran (1.0mL), beaten for 20 minutes, and then added A solution of L-tartaric acid (18.2 mg, 0.118 mmol) in tetrahydrofuran (1.0 mL) was stirred for 7 hours; suction filtered, and the filter cake was vacuum-dried at room temperature overnight to obtain a white solid (58 mg, 86.2%).
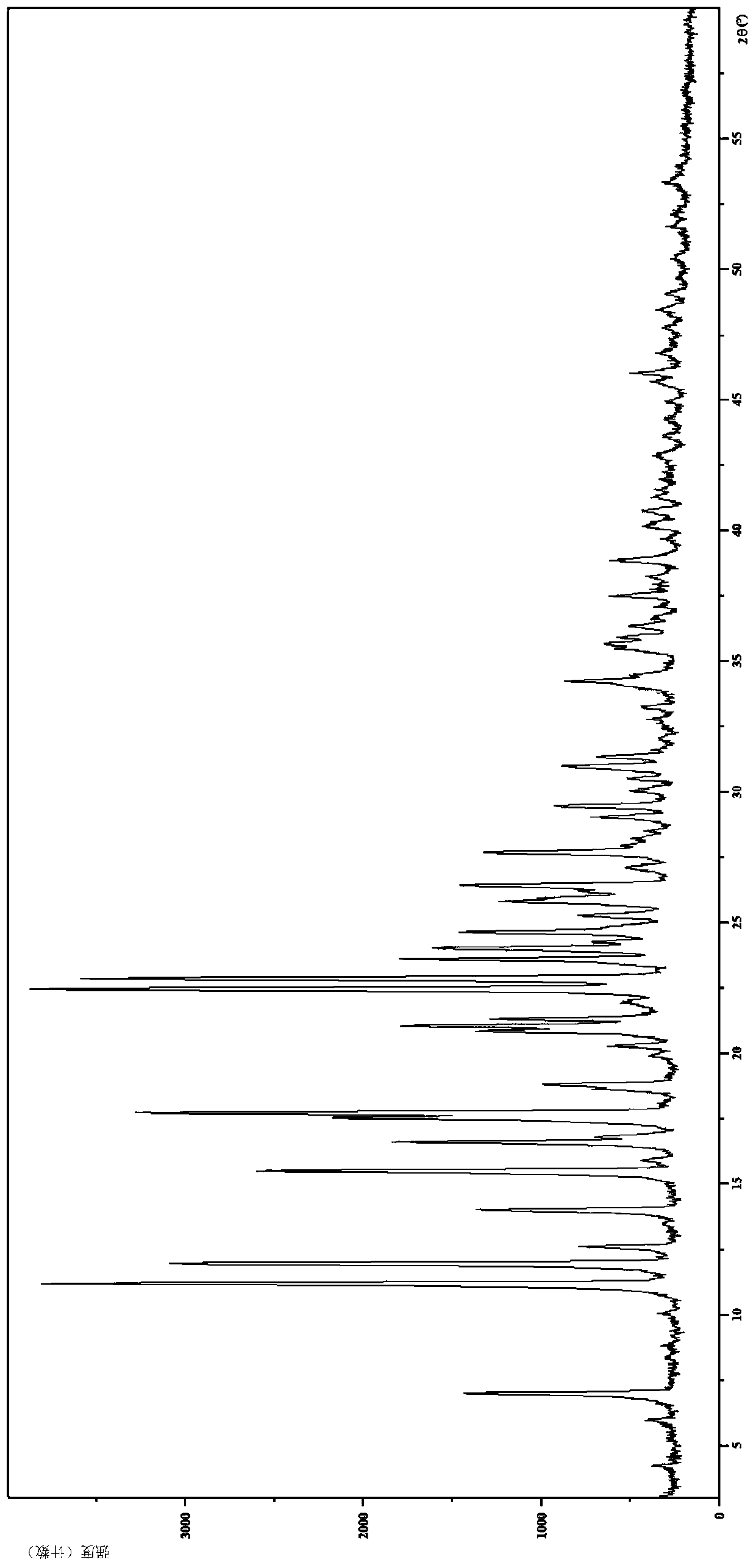
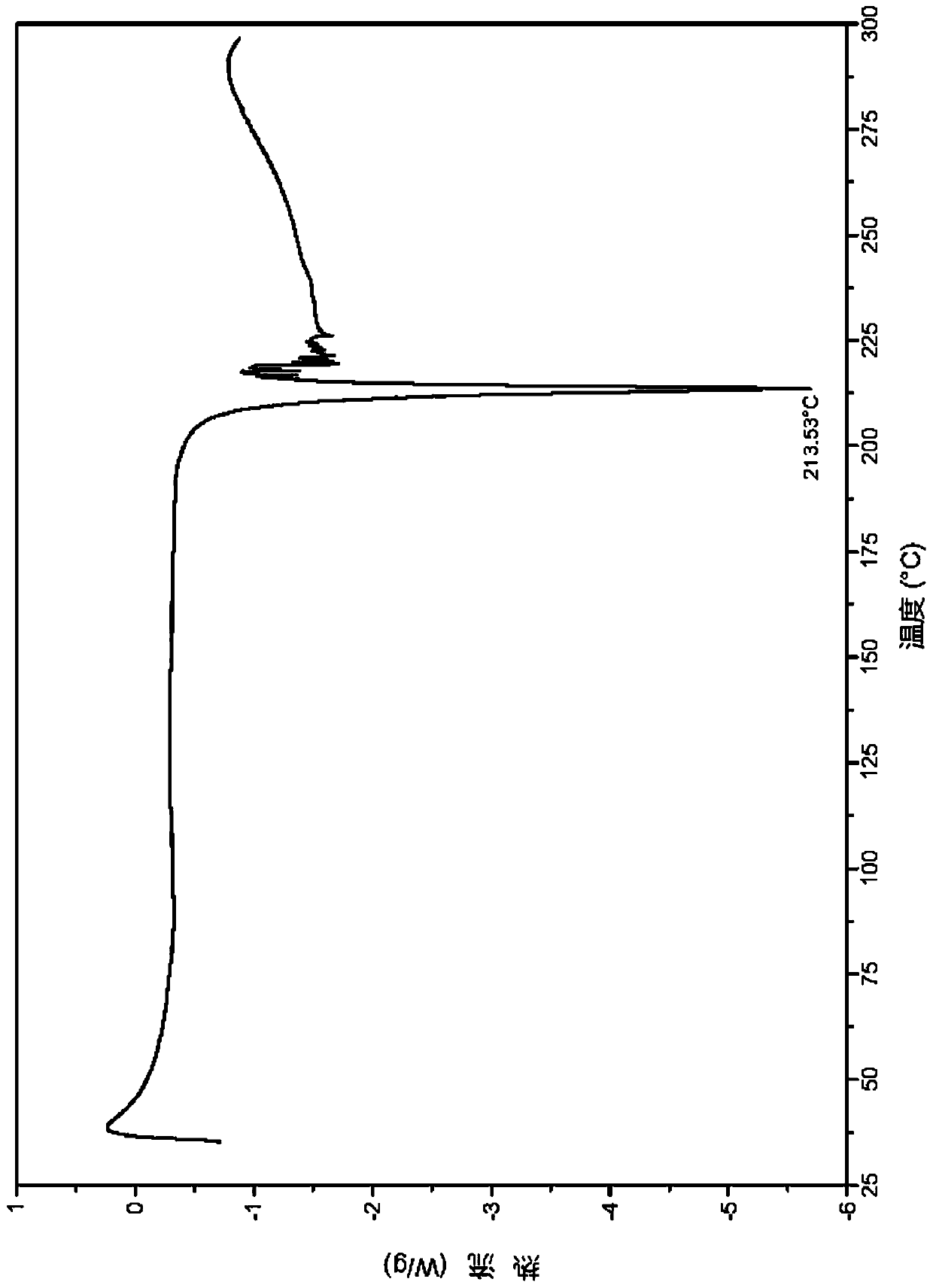
[0085] 2. Identification of L-tartrate crystal form I
[0086] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 4.17°, 5.90°, 6.96°, 7.79°, 8.72°, 10.01°, 11.14 °,11.91°,12.56°,13.95°,15.46°,15.91°,16.58°,16.81°,17.50°,17.70°,18.79°,19.89°,20.26°,20.82°,21.02°,21.29°,21.88°, 22.42°,22.82°,23...
Embodiment 2
[0088] Embodiment 2 Pharmacokinetic experiment of the present invention
[0089] Get 8-12kg male Beagle dogs and divide them into 2 groups, 3 in each group, orally administer the capsules that test samples are housed, the dose is 5mg / kg, according to the time point 0.25,0.5,1.0,2.0,4.0,6.0,9.0 and 24h blood collection. A standard curve with an appropriate range was established according to the concentration of the sample, and the concentration of the test sample in the plasma sample was determined in the MRM mode using AB SCIEX API4000 LC-MS / MS, and quantitative analysis was performed. According to the drug concentration-time curve, the pharmacokinetic parameters were calculated by WinNonLin 6.3 software non-compartmental model method. See Table 1 for details.
[0090] Table 1 PK parameters of L-tartrate salt form I
[0091] Test sample Dose (mg / kg) AUClast(h*ng / ml) Cmax(ng / ml) Tmax(h) L-tartrate salt form I 5 22600 4270 1
[0092] Experiment...
Embodiment 3
[0094] The stability experiment of embodiment 3 salts of the present invention
[0095] (1) High temperature experiment : Take an appropriate amount of the test sample and put it into a flat weighing bottle, spread it into a thin layer with a thickness of ≤5mm, place it at a temperature of 60°C for 10 days, take a sample on the 5th and 10th day, observe the color change of the sample, and test the purity of the sample by HPLC.
[0096] (2) High humidity experiment : Take an appropriate amount of a batch of test products and put them into a flat weighing bottle, spread them into a thin layer with a thickness of ≤5mm, place them for 10 days at 25°C and RH 90%±5%, take samples on the 5th and 10th days, and observe the samples The color changes, and the purity of the sample is detected by HPLC.
[0097] Experimental results:
[0098] Under the conditions of high temperature (60°C) and high humidity (25°C, RH 90%±5%), the appearance and purity of the salt of the present invent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com