Method for preparing lithium iron phosphate in ionic eutectic mixture
A technology of eutectic mixture and lithium iron phosphate, applied in phosphorus compounds, chemical instruments and methods, inorganic chemistry, etc., to achieve the effects of simple preparation, not easy to deliquescence, and avoiding high temperature restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
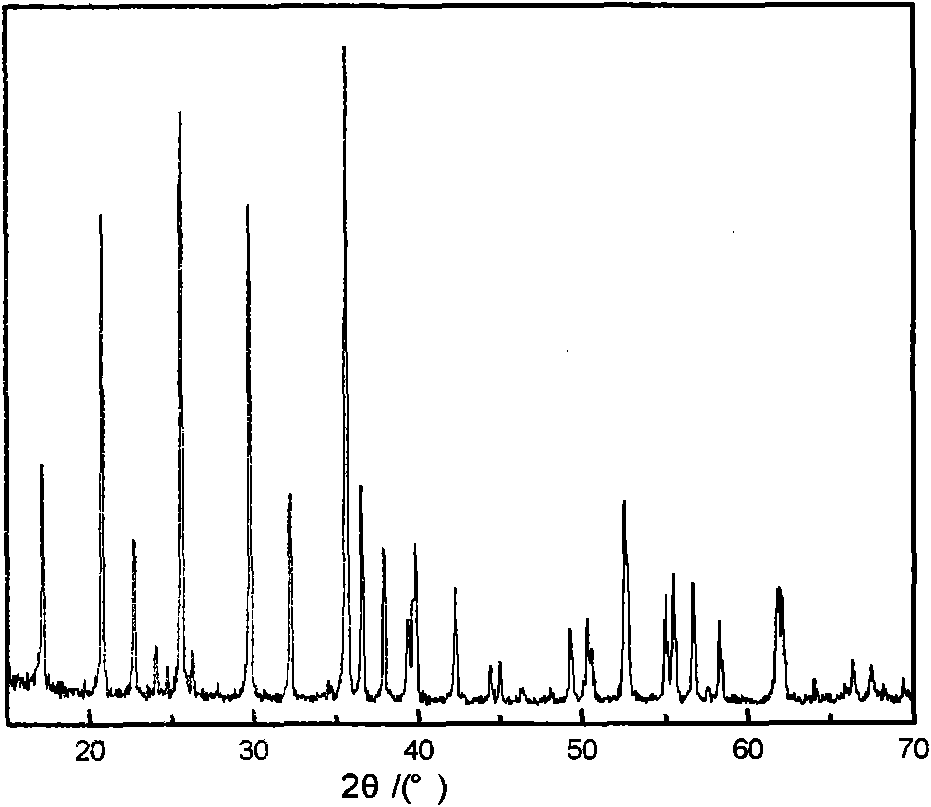
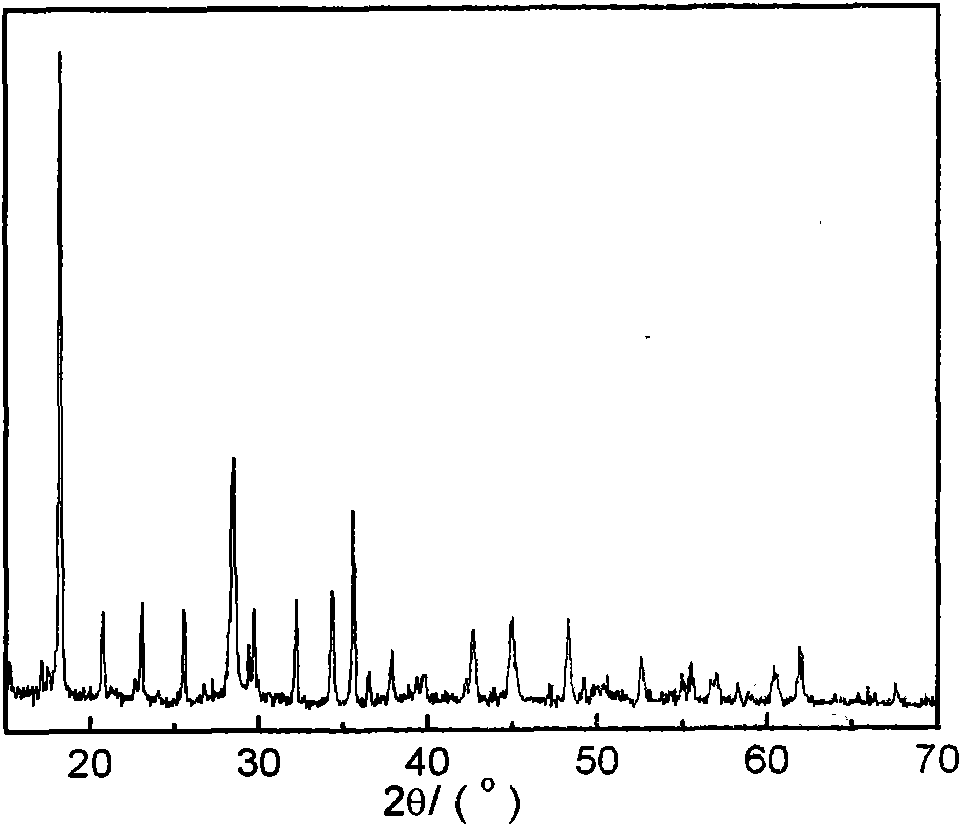
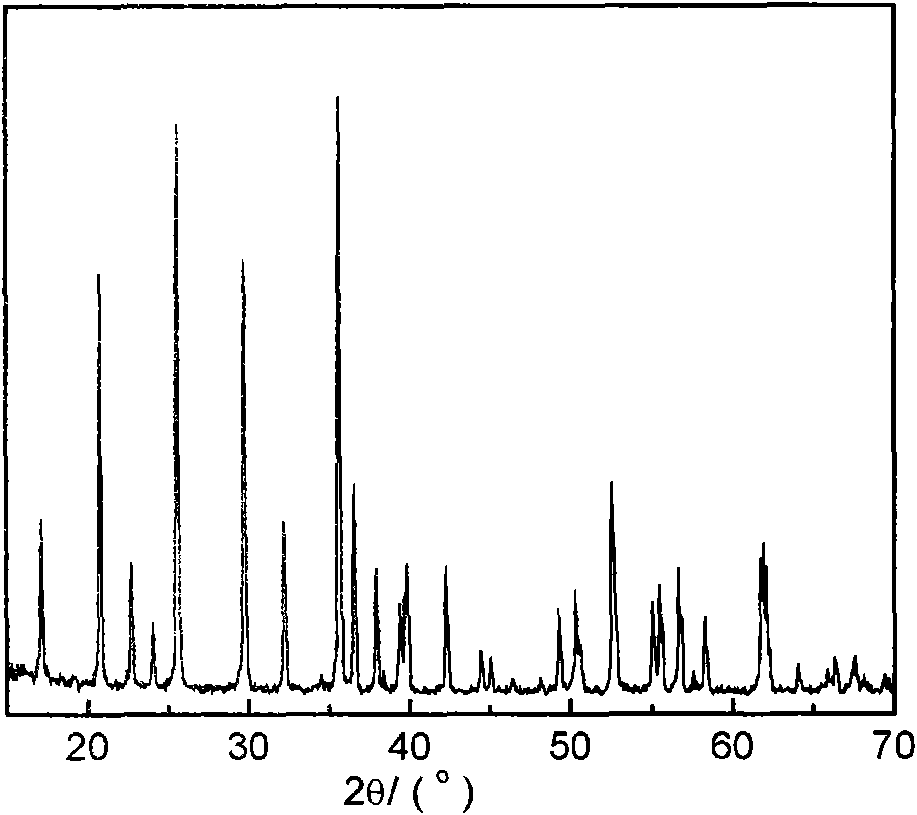
Image
Examples
Embodiment 1
[0044] 12.012g urea and 13.962g choline chloride (molar ratio 2:1) were mixed at room temperature to form a eutectic mixture, and 0.01 mole Li 2 CO 3 , 0.02 mole of ferrous oxalate, and 0.02 mole of ammonium dihydrogen phosphate are sequentially added to a stainless steel reactor lined with polytetrafluoroethylene, and the molar ratio of the reactants is Li 2 CO 3 : FeC 2 o 4 2H 2 O: NH 4 h 2 PO 4 :DES=0.5:1:1:15. After sealing, the reaction kettle was put into a homogeneous reactor at a speed of 10 rpm for crystallization reaction at 180° C. for 72 hours. After the reaction was completed, the reactor was taken out and cooled to room temperature. The resulting crystallized product was taken out, washed with alcohol and deionized water, filtered, and dried to obtain LiFePO 4 Powder.
Embodiment 2
[0046] 17.218g of imidazolone and 23.108g of choline iodide (molar ratio 2:1) were mixed at room temperature to form a eutectic mixture, followed by 0.02 mole of lithium chloride, 0.02 mole of ferrous chloride, and 0.02 mole of ammonium dihydrogen phosphate Add it to a stainless steel reactor lined with polytetrafluoroethylene, and the molar ratio of the reactants is LiCl:FeCl 2 : NH 4 h 2 PO 4 : DES = 1: 1: 1: 15, after sealing, put the reaction kettle into a homogeneous reactor at a speed of 5 rpm, and conduct a crystallization reaction at 150° C. for 120 h. After the reaction was completed, the reactor was taken out and cooled to room temperature. The resulting crystallized product was taken out, washed with alcohol and deionized water, filtered, and dried to obtain LiFePO 4 Powder.
Embodiment 3
[0048] Mix 12.670g of oxalic acid and 13.962g of choline chloride (molar ratio 1:1) at room temperature to form a eutectic mixture, add 0.02 mole of LiOH, 0.02 mole of ferrous oxalate, and 0.02 mole of diammonium hydrogen phosphate to the lining in sequence In the stainless steel reactor of polytetrafluoroethylene, the molar ratio of reactants is LiOH:FeC 2 o 4 2H 2 O:(NH 4 ) 2 HPO 4 : DES = 1: 1: 1: 10, after sealing, put the reaction kettle into a homogeneous reactor at a speed of 15 rpm, and conduct a crystallization reaction at 200° C. for 90 h. After the reaction was completed, the reactor was taken out and cooled to room temperature. The resulting crystallized product was taken out, washed with alcohol and deionized water, filtered, and dried to obtain LiFePO 4 Powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com