JAK inhibitor crystal forms, preparation methods and applications thereof
A technology of inhibitor and crystal form, applied in the field of medicine, can solve problems such as unfavorable industrial production and dosage form development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] In the preparation methods of all four crystal forms of the present invention, products prepared according to methods reported in existing literature (such as CN104262337A) are used; other solvents and reagents are commercially available chemically pure or analytically pure products.
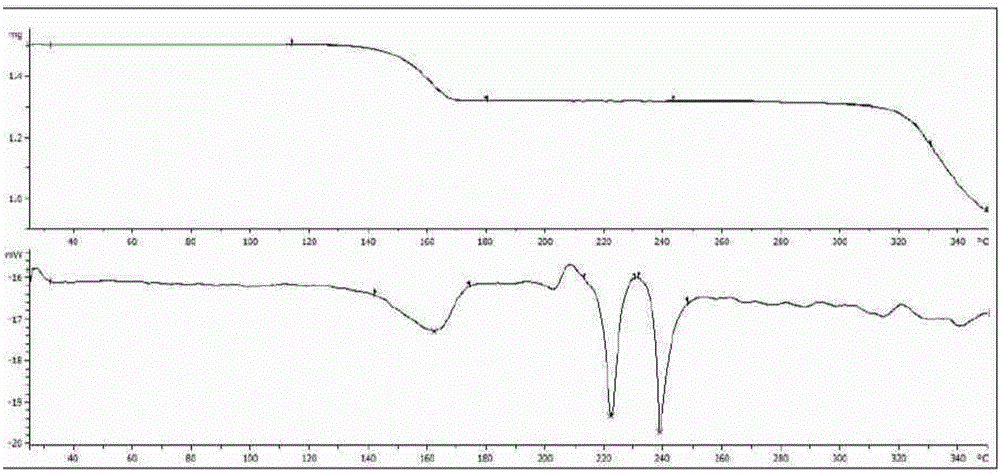
[0039] The crystal samples obtained by the crystallization method disclosed in the present invention are characterized by methods such as X-ray powder diffraction (pXRD), differential scanning calorimetry-thermogravimetric (DSC-TGA) analysis and the like.
[0040] The X-ray powder diffractometer used in the embodiment of the present invention is X'pertPRO X-ray powder diffractometer of PANalytical Company. Using Cu-Kα rays, the test power is 40kV×250mA, the scanning speed is 5° / min, and the scanning range is 4-80° (2θ) continuous scanning of θ-2θ. In the X-ray powder diffraction diagram obtained in the embodiment of the present invention, the horizontal axis represents the 2θ position of ...
Embodiment 1
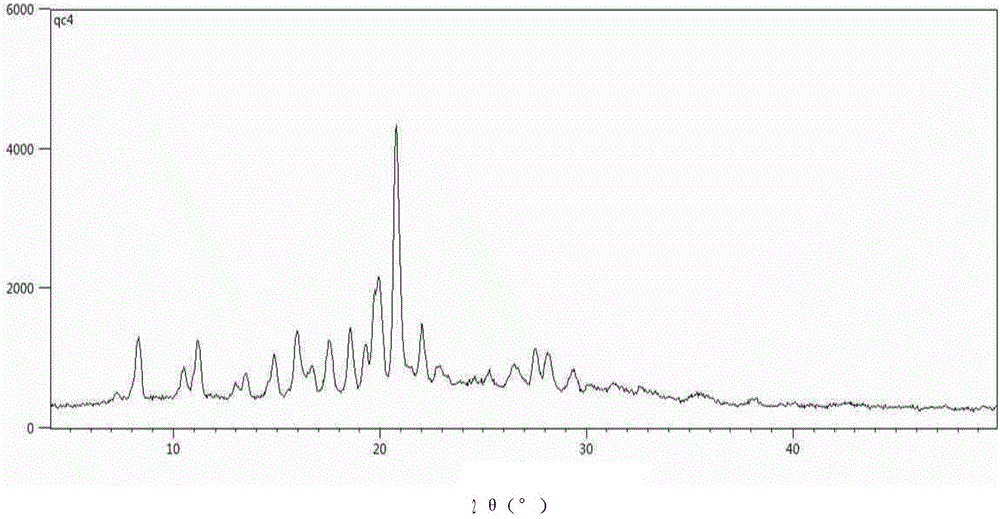
[0042] Example 1 Preparation and characterization of crystal form H1.
[0043] 0.5 g of N-(5-(4-(1,1-dioxothiomorpholinyl)methyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridine- 2-yl) cyclopropanecarboxamide and 0.1 g of water are added to 5 ml of 1,4-dioxane solvent, and the mixture is heated to 85±2°C and stirred to completely dissolve the solid, followed by a speed of 1 to 3°C / min Slowly cool to room temperature and stand at room temperature for 48 hours, and the precipitated white powdery crystals are taken out by filtration, which is the crystal form H1.
[0044] Powder X-ray diffraction analysis of the crystal form H1 obtained in Example 1:
[0045] The crystal form H1 obtained in Example 1 was further ground, and subjected to powder X-ray diffraction analysis. The results are shown in the attached figure 1 shown. attached figure 1 The X-ray diffraction data of the corresponding crystal form H1 are shown in Table 1.
[0046] Table 1 Powder X-ray Diffraction Analysis of Form ...
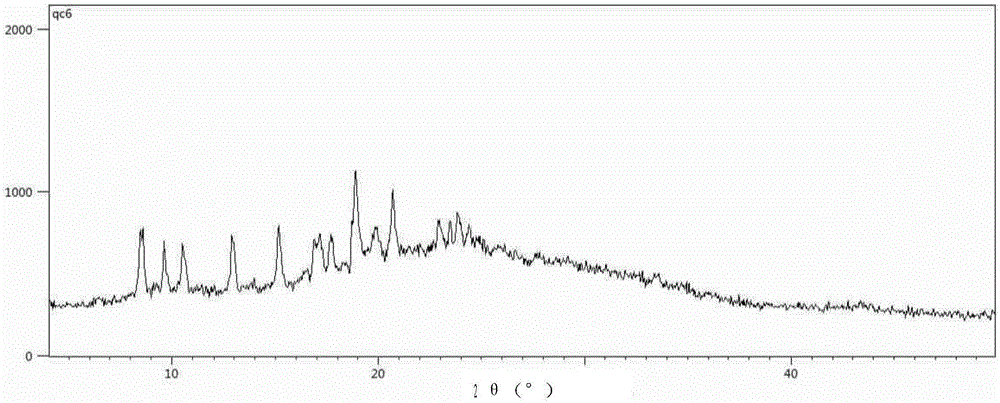
Embodiment 2
[0052] Example 2 Preparation of Form H1.
[0053] 0.5 g of N-(5-(4-(1,1-dioxothiomorpholinyl)methyl)phenyl)-[1,2,4]triazolo[1,5-a]pyridine- 2-yl) cyclopropanecarboxamide and 0.5 g of water are added to 10 ml of 1,4-dioxane solvent, and the mixture is heated to 80±2°C and stirred to completely dissolve the solid, followed by a speed of 1 to 3°C / min After slowly cooling to room temperature and standing at room temperature for 96 hours, the precipitated white powdery crystals were taken out by filtration. It was analyzed by powder X-ray diffraction and confirmed to be the crystal form H1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com