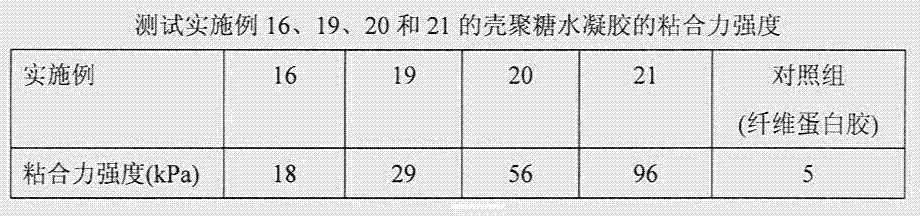
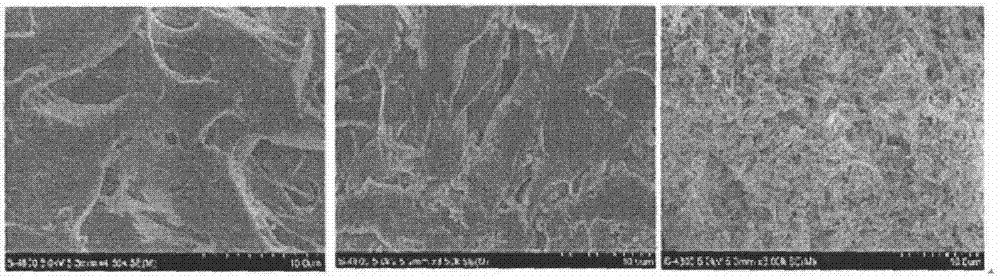
Chitosan hydrogel, and preparation method and application thereof
A technology of chitosan and sugar water, applied in the field of biomedical materials, can solve the problems of poor mechanical properties, high brittleness and poor water solubility of chitosan, and achieve the effects of low mechanical strength, fast degradation rate and soft physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
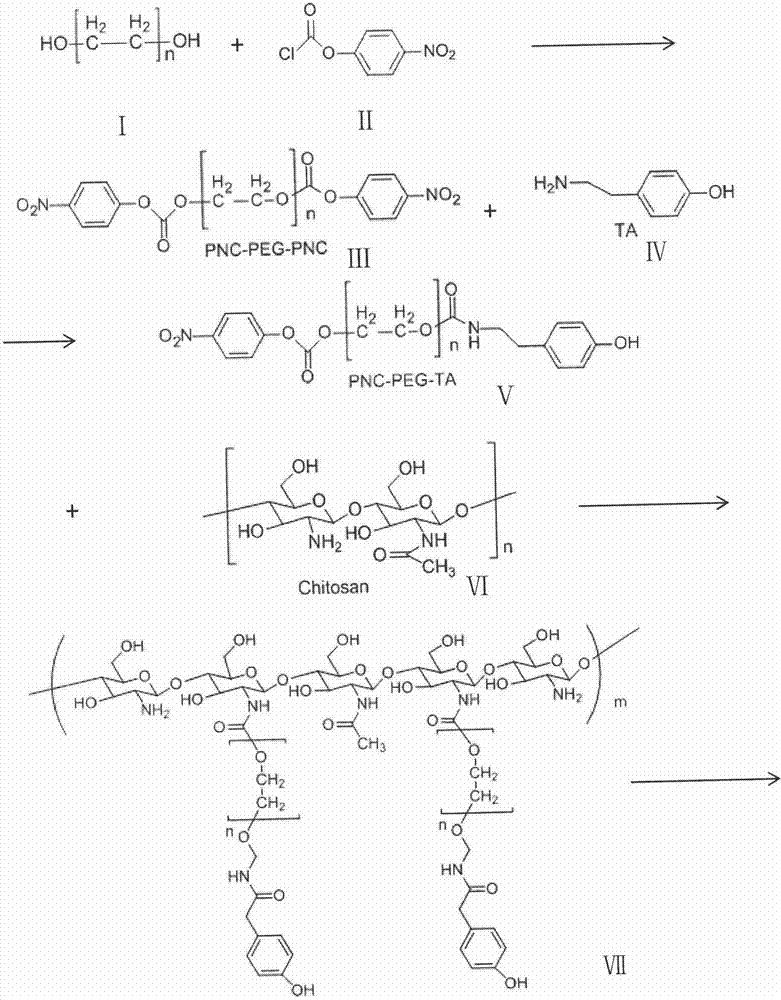
[0056] Embodiment 1: the preparation of PNC-PEG-PNC (III)
[0057] Polyethylene glycol (I) (PEG, 10.0 g, molecular weight 3000, monomer molecular weight 62, hydroxyl mole number 5 mmol) was dissolved in 100 mL of dichloromethane. 4-Dimethylaminopyridine (0.916 g, 7.5 mmol) and triethanolamine (0.759 g, 7.5 mmol) dissolved in 20 mL of dichloromethane were added under nitrogen protection. After stirring for 15-20 min, p-nitrobenzene chloroformate (II) (PNC) (1.511 g, 7.5 mmol) dissolved in 50 mL of dichloromethane was added to the solution. Stir the reaction at room temperature under the protection of nitrogen for 12 hours; remove most of the dichloromethane by rotary evaporation, add anhydrous ether for sedimentation, and filter to obtain a white solid. The solid was washed three times with anhydrous ether, and dried in vacuo to obtain a white solid powder with a yield of 82.1%.
Embodiment 2
[0058] Embodiment 2: the preparation of PNC-PEG-PNC (III)
[0059] Polyethylene glycol (I) (PEG, 10.0 g, molecular weight 4000, monomer molecular weight 62, hydroxyl mole number 5 mmol) was dissolved in 50 mL of dichloromethane. 4-Dimethylaminopyridine (0.916 g, 7.5 mmol) and triethanolamine (0.759 g, 7.5 mmol) dissolved in 10 mL of dichloromethane were added under nitrogen protection. After stirring for 15-20 min, p-nitrobenzene chloroformate (II) (PNC) (1.511 g, 7.5 mmol) dissolved in 25 mL of dichloromethane was added to the solution. Stir the reaction at room temperature under the protection of nitrogen for 24 hours; remove most of the dichloromethane by rotary evaporation, add anhydrous ether for sedimentation, and filter to obtain a white solid. The solid was washed three times with anhydrous ether, and dried in vacuo to obtain a white solid powder with a yield of 79.1%.
Embodiment 3
[0060] Embodiment 3: the preparation of PNC-PEG-PNC (III)
[0061] Polyethylene glycol (I) (PEG, 10.0 g, molecular weight 5000, monomer molecular weight 62, hydroxyl mole number 5 mmol) was dissolved in 150 mL of dichloromethane. 4-Dimethylaminopyridine (1.221 g, 10 mmol) and triethanolamine (1.102 g, 10 mmol) dissolved in 30 mL of dichloromethane were added under nitrogen protection. After stirring for 15-20 min, p-nitrobenzene chloroformate (II) (PNC) (2.015 g, 10 mmol) dissolved in 30 mL of dichloromethane was added to the solution. Stir the reaction at room temperature under the protection of nitrogen for 36 hours; remove most of the dichloromethane by rotary evaporation, add anhydrous ether for sedimentation, and filter to obtain a white solid. The solid was washed three times with anhydrous ether, and dried in vacuo to obtain a white solid powder with a yield of 83.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com