Pharmaceutical composition for treating small cell lung cancer and application, kit and package thereof
A small cell lung cancer and kit technology, applied in drug combination, packaging, drug delivery, etc., can solve problems such as unsatisfactory curative effect, second-line treatment, patient recurrence, etc., and achieve excellent therapeutic effect and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
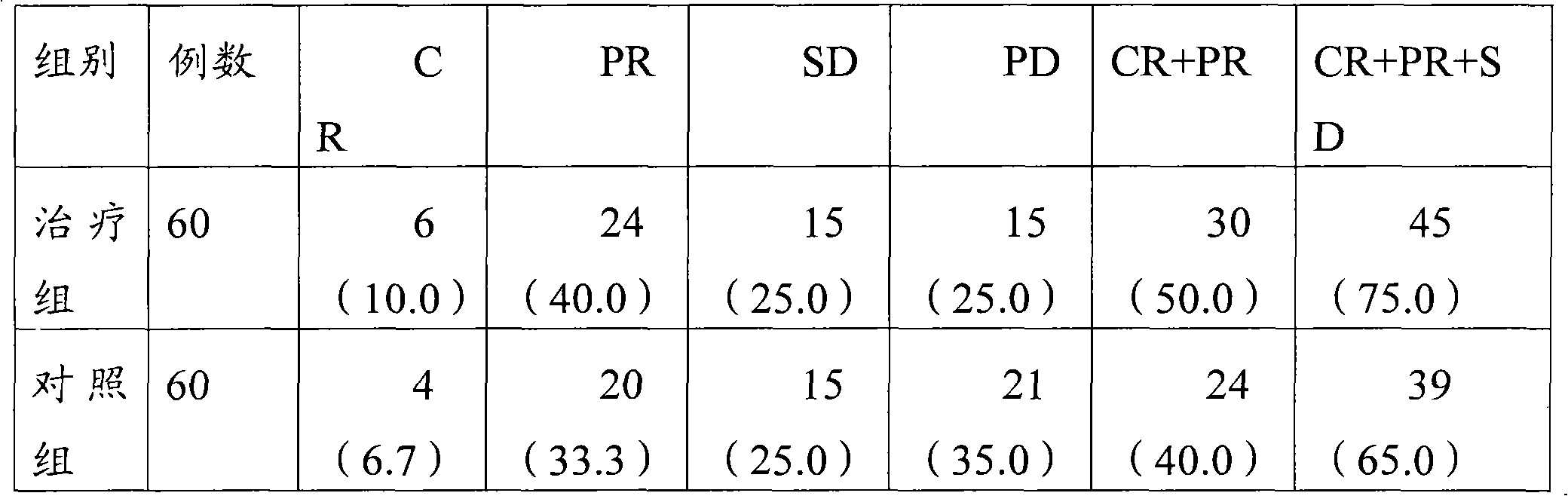
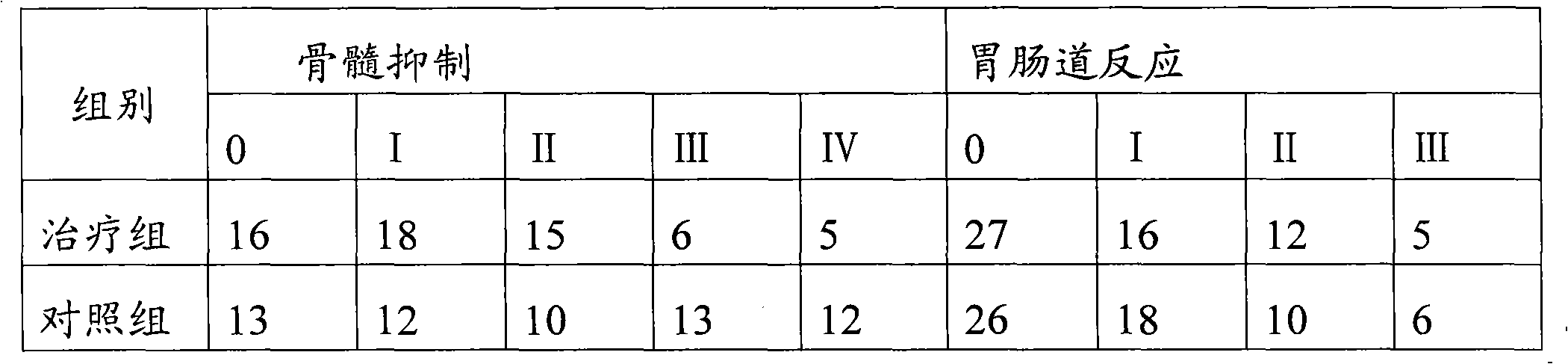
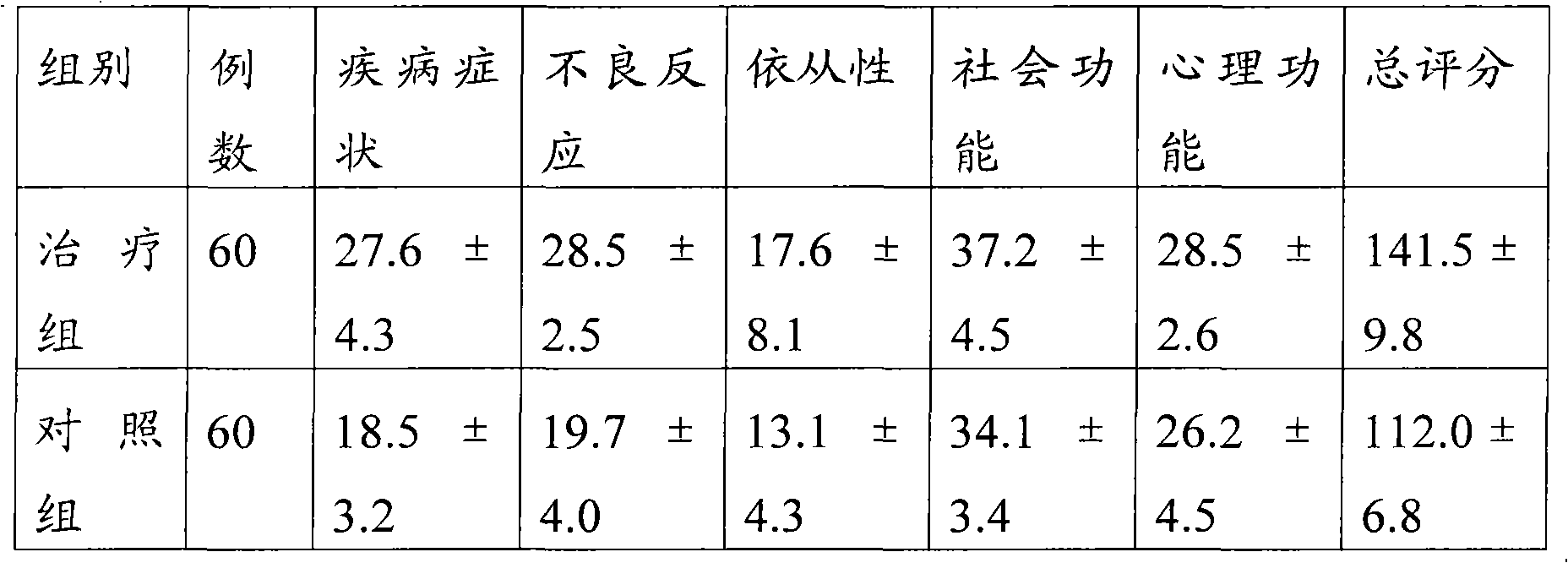
[0035] Example 1 This experiment compared and observed the effectiveness and safety of lobaplatin freeze-dried combined with etoposide injection and lobaplatin freeze-dried single product in the treatment of extensive-stage small cell lung cancer.
[0036] Drugs used:
[0037] 1. Lobaplatin (LBP) for injection: 10mg / bottle, white lyophilized powder, batch number 20070101, each bottle contains 10mg lobaplatin in anhydrous form; produced by Hainan Changan International Pharmaceutical Co., Ltd.;
[0038] 2. Etoposide injection (VP-16): the specification is 5ml: 100mg, colorless to light yellow clear liquid, excipients are polyethylene glycol 400, absolute ethanol, Tween-80, citric acid, each A 5ml injection contains 100mg etoposide; produced by Qilu Pharmaceutical Co., Ltd.
[0039] Treatment plan and treatment effect evaluation plan:
[0040] 1. General Information
[0041] 120 patients with extensive stage SCLC were diagnosed by pathology and randomly divided into two groups...
Embodiment 2
[0073] Example 2 This experiment compared and observed the effectiveness and safety of lobaplatin freeze-dried combined with etoposide injection and cisplatin freeze-dried combined with etoposide injection for the first-line treatment of small cell lung cancer.
[0074] Drugs used:
[0075] 1. Lobaplatin (LBP) for injection: 10mg / bottle, white lyophilized powder, batch number 20070101, each bottle contains 10mg lobaplatin in anhydrous form; produced by Hainan Changan International Pharmaceutical Co., Ltd.;
[0076] 2. Etoposide injection (VP-16): 5ml: 100mg / bottle, colorless to light yellow clear liquid, excipients are polyethylene glycol 400, absolute ethanol, Tween-80, citric acid, each 5ml injection contains 100mg etoposide; produced by Qilu Pharmaceutical Co., Ltd.
[0077] 3. Cisplatin (DDP) for injection: 10mg / bottle; yellow powder, batch number 36-014050304, excipients: mannitol, sodium chloride. Each bottle contains cisplatin 10mg in anhydrous form, produced by Jiang...
experiment example 3
[0111] Experimental example 3 Evaluation experiment of the effect of changes in the dosage of lobaplatin and etoposide on the therapeutic effect
[0112] Select 40 patients with extensive-stage SCLC diagnosed by pathology, with an average weight of (70.0±0.5) KG, an average height of (1.66±0.15) meters, and an average body surface area of (1.61±0.22) m 2 , by dose escalation (dose climbing) test, determine the optimal dose of LBP combined with VP-16 for the treatment of SCLC (the LBP and VP-16 used are the same as in Example 1), evaluate the effective rate of treatment, disease control rate, and safety as Check indicators. Dose-limiting toxicity (DLT) is defined as: ANC (neutropenia), PLT (thrombocytopenia) or anemia of CTCAE grade 4 in the first cycle of the subject, or CTCAE grade 3 or 4 (except nausea, vomiting and alopecia) Hematological toxicity. VP-16 and LBP are increased from low doses respectively, divided into 4 grades, VP-16 respectively: 90mg / m 2 (average dail...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Body surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com