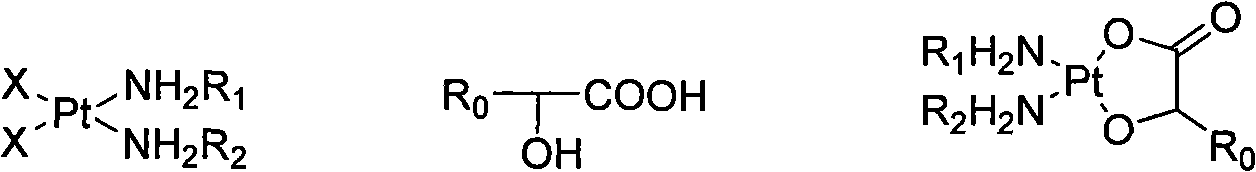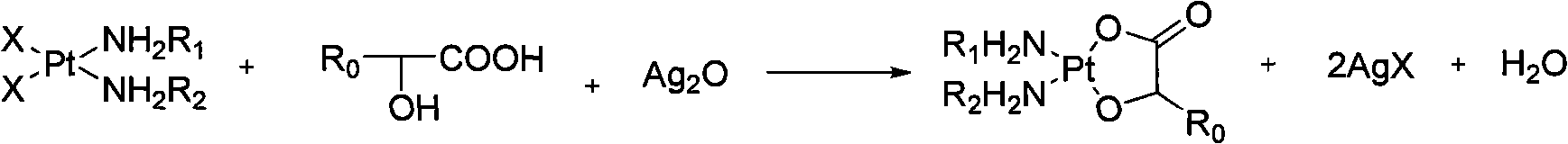Method for preparing hydroxy carboxylic acid platinum complexes
A platinum complex and hydroxycarboxylic acid technology, applied in the field of hydroxycarboxylic acid platinum complexes, can solve the problems of low yield and complicated production process, and achieve the effects of high yield, short process and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1--the preparation of nedaplatin
[0024] Feeding:
[0025] Platinum diiododiammine 4.83g 0.01mol
[0026] Silver oxide 2.30g 0.01mol
[0027] Glycolic acid 0.80g 0.01mol
[0028] 150ml deionized water
[0029] Add the above items into a 250ml one-necked bottle, and react with electromagnetic stirring at room temperature for 16 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure at 60°C. Cool, wash with water, filter, wash with ethanol, and dry under vacuum at 40°C for 2 to 3 hours to obtain 1.85 g of the product nedaplatin, with a yield of 61.1% and a content of 99.7%.
[0030] The obtained product is consistent with the target product through elemental analysis:
[0031] Elemental analysis:
[0032] PtC 2 h 5 N 2 o 3 M=303.18
[0033] Theoretical value: C: 7.92% H: 2.64% N: 9.24% Pt: 64.36%
[0034] Found: C: 7.90% H: 2.63% N: 9.25% Pt: 64.33%
Embodiment 2
[0035] Embodiment 2--the preparation of nedaplatin
[0036] Feeding:
[0037] Platinum diiododiammine 4.83g 0.01mol
[0038] Silver oxide 2.30g 0.01mol
[0039] Glycolic acid 0.80g 0.01mol
[0040] 150ml deionized water
[0041] The above items were added into a 250ml single-necked bottle, and reacted with electromagnetic stirring at 60°C for 6 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure at 60°C. Cool, wash with water, filter, wash with ethanol, and dry under vacuum at 45° C. for 2 to 3 hours to obtain 2.05 g of the product nedaplatin, with a yield of 67.7% and a content of 99.6%.
[0042] The obtained product is consistent with the target product through elemental analysis:
[0043] Elemental analysis:
[0044] PtC 2 h 5 N 2 o 3 M=303.18
[0045] Theoretical value: C: 7.92% H: 2.64% N: 9.24% Pt: 64.36%
[0046] Found: C: 7.89% H: 2.62% N: 9.23% Pt: 64.31%
Embodiment 3
[0047] Embodiment 3--the preparation of lobaplatin
[0048] Feeding:
[0049] cis-[trans-1,2-cyclobutylbis(methylamine)-N,N]-diiodoplatinum 5.63g 0.01mol
[0050] Silver oxide 2.30g 0.01mol
[0051] L-lactic acid 0.90g 0.01mol
[0052] 150ml deionized water
[0053] Add the above items into a 250ml one-necked bottle, and react with electromagnetic stirring at 40°C for 10 hours. After filtration, the filtrate was evaporated to dryness under reduced pressure at 60°C. Cool, wash with water, filter, wash with ethanol, and vacuum-dry at 40°C for 2 to 3 hours to obtain 2.65 g of lobaplatin, with a yield of 58.8% and a content of 99.6%.
[0054] The obtained product is consistent with the target product through elemental analysis:
[0055] Elemental analysis:
[0056] PtC 9 h 24 N 2 o 6 M=451.38
[0057] Theoretical value: C: 23.95% H: 5.36% N: 6.21% Pt: 43.22%
[0058] Found: C: 23.93% H: 5.33% N: 6.18% Pt: 43.19%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com