Lobaplatin pharmaceutical composition for injection and preparation method thereof
A technology for composition and injection, which is applied in the direction of drug combinations, pharmaceutical formulas, active ingredients of heavy metal compounds, etc., can solve the problems of high content of related substances and moisture in freeze-dried finished products, long preparation process time, and large damage to production equipment. The effect of reducing complicated operation process, improving appearance and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] prescription
[0042] Loba5g lactose15g Water for Injection Volume up to 300ml
[0043] Preparation Process:
[0044] 1. Weigh 15g of lactose, add 240ml of water for injection at room temperature, and stir until completely dissolved;
[0045] 2. Weigh 5 g of lobaplatin trihydrate, add it to the lactose solution, and stir until completely dissolved;
[0046] 3. Add 0.15g of activated carbon to the solution, decarburize at 40°C for half an hour, filter and decarburize; add water for injection to the full amount, and mix well;
[0047] 4. Fill the liquid medicine in a unit vial and send it to the freeze-drying box for freeze-drying;
[0048] 5. After the product enters the box, it is cooled to about -45°C and pre-frozen and kept for 2.5 hours; the cold trap is opened and the vacuum is turned on; the temperature is raised to 35°C and then kept for 20 hours;
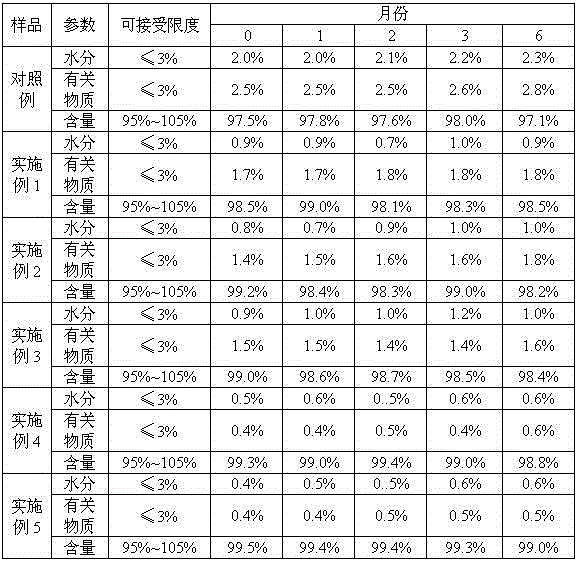
[0049] The finished product has 1.7% related substances, 0.9% moisture and 98.5% content.
Embodiment 2
[0051] prescription
[0052] Loba10g Mannitol1g Water for Injection Constant volume to 100ml
[0053] Preparation Process:
[0054] 1. Weigh 1g of mannitol, add 70ml of water for injection at room temperature, and stir until completely dissolved;
[0055] 2. Weigh 10g of lobaplatin, add it to the mannitol solution, and stir until completely dissolved;
[0056] 3. Add 0.1g activated carbon to the solution, decarburize at 30°C for half an hour, filter and decarburize; add water for injection to the full amount, and mix well;
[0057] 4. Fill the liquid medicine in a unit vial and send it to the freeze-drying box for freeze-drying;
[0058] 5. After the product enters the box, it is cooled to about -45°C and pre-frozen and kept for 2.5 hours; the cold trap is opened and the vacuum is turned on; the temperature is raised to 35°C and then kept for 18 hours;
[0059] The finished product has 1.4% related substances, 0.8% moisture and 99.2% content.
Embodiment 3
[0061] prescription
[0062] Loba10g glucose50g Water for Injection Volume up to 200ml
[0063] Preparation Process:
[0064] 1. Weigh 50g glucose, add 140ml water for injection at room temperature, and stir until completely dissolved;
[0065] 2. Weigh 10g lobaplatin, add it to the glucose solution, and stir until it is completely dissolved;
[0066] 3. Add 0.06g of activated carbon to the solution, decarburize at 50°C for half an hour, filter and decarburize; add water for injection to the full amount and mix well;
[0067] 4. Fill the liquid medicine in a unit vial and send it to the freeze-drying box for freeze-drying;
[0068] 5. After the product enters the box, cool down to about -45°C and pre-freeze and keep it for 2.5 hours; open the cold trap and open the vacuum; increase the temperature to 5°C and then keep it for 10 hours; increase the temperature to 38°C and keep it warm for 15 hours; , Get the finished product out of the box.
[0069] The finished product has 1.5% rel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com