Lobaplatin crystal, and preparation method thereof and pharmaceutical application thereof
A lobaplatin and crystal technology, which is applied to new crystal forms and their preparation methods and application fields in medicines, can solve the problems of difficulty in preparing preparations and poor stability, and achieves high fluidity, good stability and high solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] On the other hand, the present invention provides a method for preparing a new crystal form of lobaplatin that is simple to prepare, easy to operate, and suitable for scale-up production.
[0048] In a preferred embodiment, the preparation method of lobaplatin new crystal form E of the present invention comprises the following steps:
[0049] a. Preparation of lobaplatin dihydrate: Weigh lobaplatin trihydrate in a container, add 15-30ml of organic solvent, suspend and stir at room temperature for 45-50h, filter, wash with ether, and obtain a white powder after vacuum drying, namely Lobaplatin dihydrate;
[0050] Wherein, the organic solvent is selected from methyl tert-butyl ether, toluene, diethyl ether, butyl acetate, 1,4-dioxane or n-heptane.
[0051] b. Preparation of the target crystal form: Weigh the lobaplatin dihydrate obtained in step a, place it in a container, add ethylene glycol dimethyl ether, suspend and stir at room temperature for 45-50 hours, precipita...
Embodiment 1
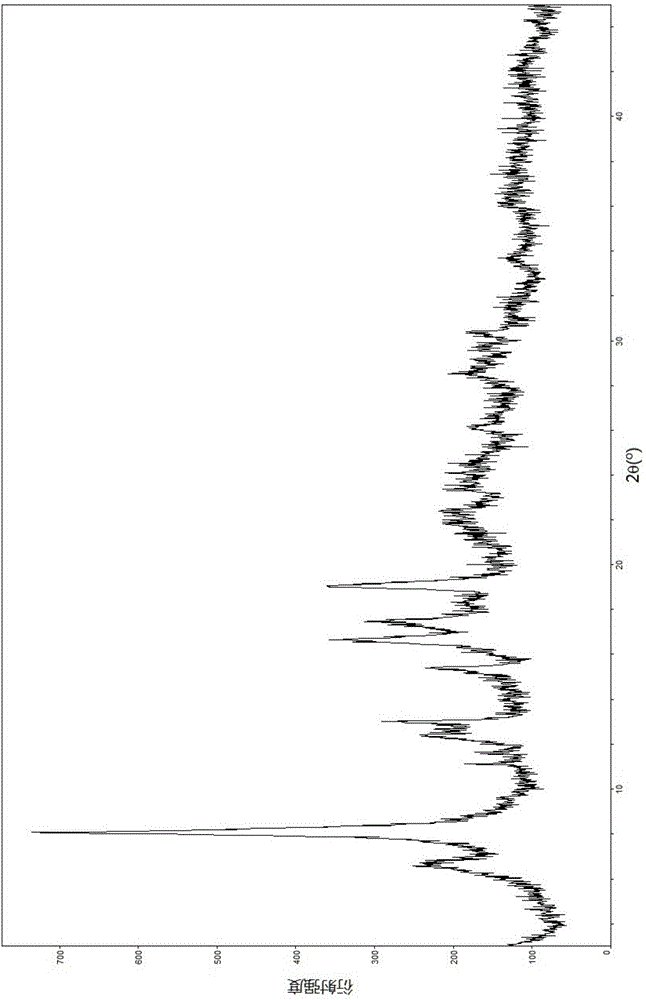
[0055] Embodiment 1: Screening analysis of each crystal form
[0056] 1.1 Screening by room temperature volatile crystallization method
[0057] Take 20 mg of lobaplatin trihydrate sample and put it into a 10 ml sample bottle, add 3 ml of absolute ethanol or absolute methanol, after fully dissolving, place it in an environment of 25°C and slowly volatilize to obtain a dry solid, which is then determined by PXRD. The results are shown in Table 2 below:
[0058] Table 2 normal temperature volatilization and crystallization test results
[0059] Numbering
PXRD (possible crystal number)
1-1
Absolute ethanol
B
1-2
B
[0060] The results showed that the crystal forms obtained in absolute methanol and absolute ethanol were compared and found to be the same crystal form, tentatively named as crystal form B.
[0061] 1.2 Screening by suspension crystallization method
[0062] Take 20mg of lobaplatin t...
Embodiment 2
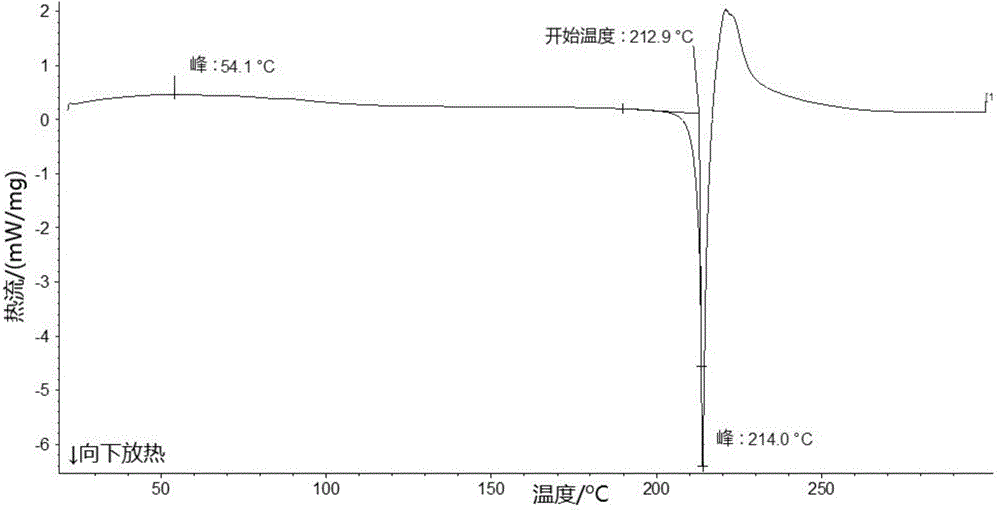
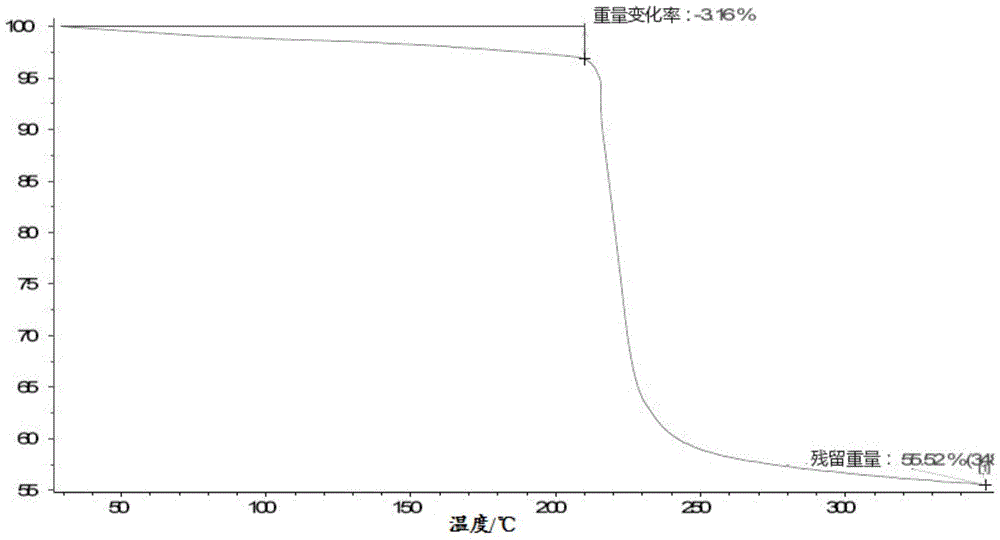
[0104] Example 2: Preparation of a new crystal form of lobaplatin named as crystal form E
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com