Novel synthesis method of lobaplatin intermediate
An intermediate, a new synthesis technology, applied in organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of harsh reaction conditions, large environmental pollution, low yields, etc., to achieve mild reaction conditions, reduced Production cost and yield improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
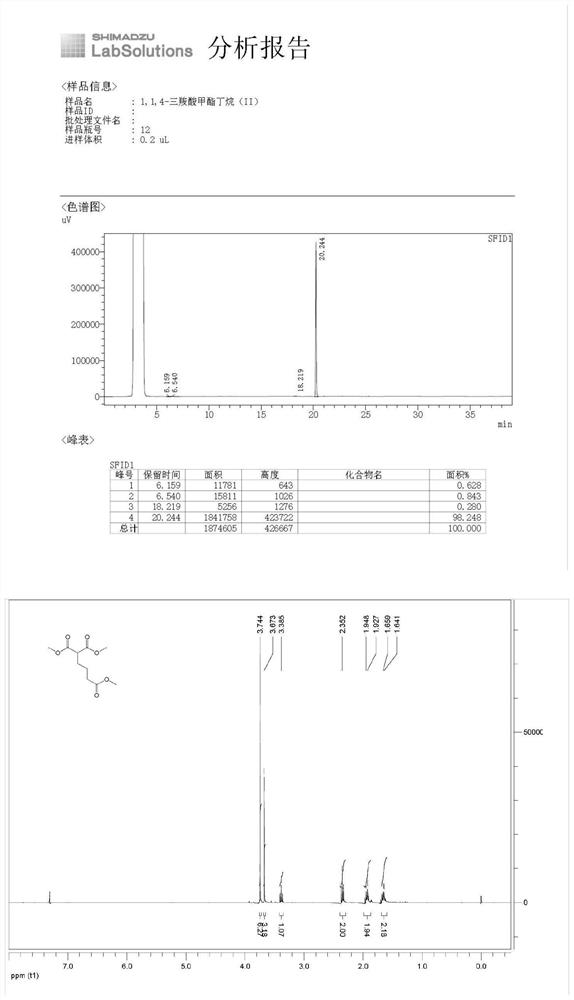
[0038] The synthesis of 1,1,4-tricarboxylic acid methyl ester butane (II), the reaction formula is as follows:
[0039]
[0040] Concrete preparation process is as follows:
[0041] (1) Under nitrogen protection, put N,N'-dimethylformamide (500mL), dimethyl malonate (50.00g, 0.378mol) and anhydrous potassium carbonate (78.46g, 0.568mol) into the reaction flask and methyl 4-bromobutyrate (82.22 g, 0.454 mol). Stir the reaction at room temperature. After GC detection detects that the raw material dimethyl malonate disappears, add 2N hydrochloric acid solution, and then extract with ethyl acetate (250mL*3). The obtained organic phases are combined and washed with saturated brine. Dry over sodium sulfate, filter and concentrate to give the product 1,1,4-tricarboxylic acid methyl butane (II) (86.15 g, yield 98%) as a colorless transparent liquid.
[0042] (2) GC and nuclear magnetic analysis are carried out to product, wherein, GC analysis result is as shown in table 1, and nu...
Embodiment 2
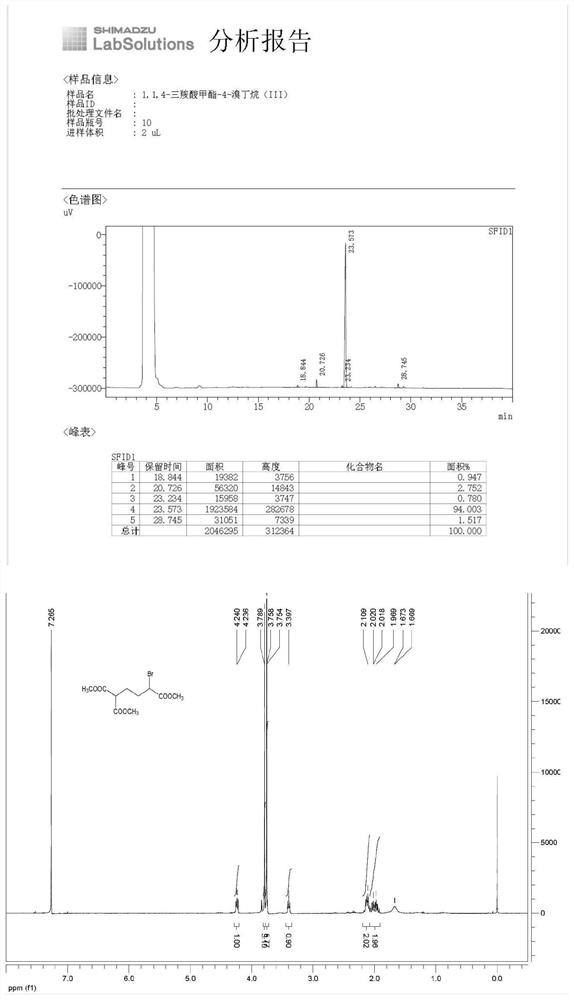
[0046] The synthesis of 1,1,4-methyl tricarboxylate-4-bromobutane (III), the reaction formula is as follows:
[0047]
[0048] Concrete preparation process is as follows:
[0049](1) Add 1,1,4-tricarboxylic acid methyl butane (II) (80.00g, 0.344mol) and dichloromethane (800mL) into the reaction flask under nitrogen protection, and cool to -10°C-0 ℃, add dibromohydantoin (103.42g, 0.362mol) in dichloromethane (200mL) dropwise, after the dropwise addition, continue to control the temperature at 10-20℃ and stir the reaction, the raw material 1,1,4-tricarboxylate is detected by GC After methyl butane(II) / 1,1,4-tricarboxylic acid methyl-4-bromobutane(III)<5%, the reaction is over, add water to quench the reaction, separate the liquid, and use the water phase again Dichloromethane (200mL*2) was extracted, and the obtained organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain a light yellow transparent ...
Embodiment 3
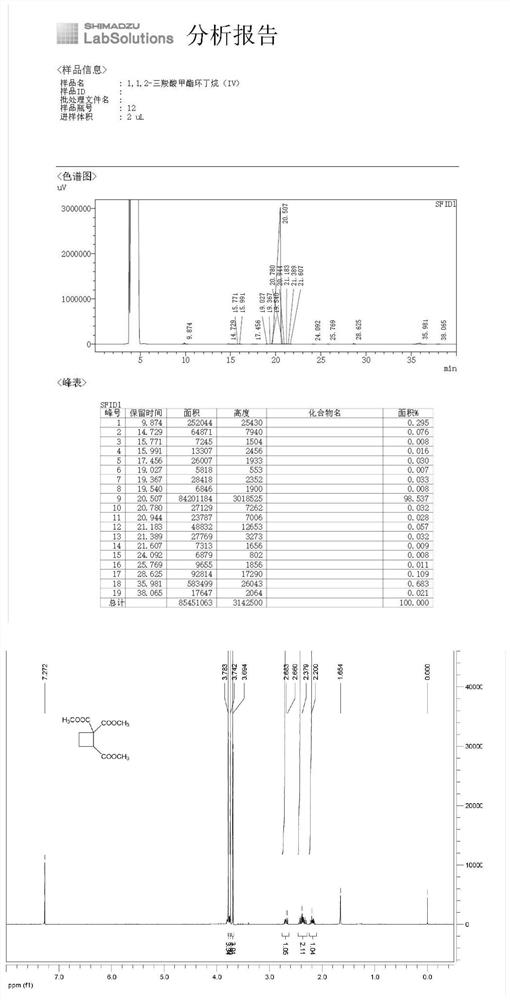
[0054] The synthesis of 1,1,2-tricarboxylic acid methyl cyclobutane (IV), the reaction formula is as follows:
[0055]
[0056] Concrete preparation process is as follows:
[0057] (1) Methyl 1,1,4-tricarboxylate-4-bromobutane (III) (85.00g, 0.273mol), N,N'-dimethylformamide (850mL) and carbonic acid Potassium (75.52g, 0.546mol) was added to the reaction flask, and the reaction was stirred at 20°C-30°C. GC detected that the raw material 1,1,4-tricarboxylic acid methyl ester-4-bromobutane (III) disappeared, and the reaction was completed. Add water to quench the reaction, and then extract with dichloromethane. The obtained organic phase is successively washed with 2N hydrochloric acid solution, water and saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain the product brownish-yellow transparent liquid, the crude product 1,1,2-Tricarboxylate methyl cyclobutane, purified by distillation, collect the desired product fraction (collection te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com