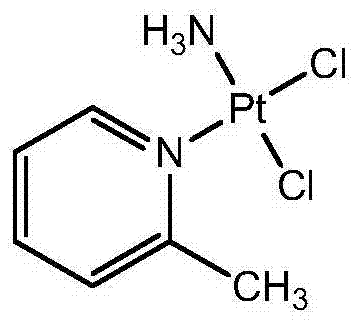
Stabilized picoplatin oral dosage form
A dosage form, the technology of picoplatin, used in the field of cancer treatment, can solve problems such as low stability, uncomfortable infusion, and deformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] containing TiO 2 or CaSO 4 Impurities formed from picoplatin in solutions of
[0107] Picoplatin solution and TiO 2 solution, pure OPADRY (without TiO 2 ) solution, and containing TiO 2 or CaSO 4 Mix with the standard coating OPADRY solution. After standing, the solution was analyzed by HPLC for the amount of picoplatin degradation products 2-picoline and trichloroplatinate (TCAP). The results are shown in Table 1.
[0108] Table 1
[0109]
[0110] show CaSO 4 OPADRY coating does not cause picoplatin to occur as in TiO 2 or TiO 2 Degradation observed in OPADRY products,.
Embodiment 2
[0112] Fe as a function of time 2+ Effect of Concentration on TCAP Formation from Picoplatin
[0113] Preparation of FeSO 4 solution, and it was added to the aqueous solution of picoplatin to obtain Fe as shown in the table below 2+ the final concentration. The percentage of TCAP formed by conversion of picoplatin was determined by HPLC at the indicated time points and is shown in Table 2 in percent.
[0114] Table 2
[0115] Fe 2+ (ppm)
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com