Oral formulations for picoplatin
A preparation, the technology of picoplatin, applied in this field, can solve the problems of low solubility, uncomfortable infusion, difficult to prepare oral dosage forms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
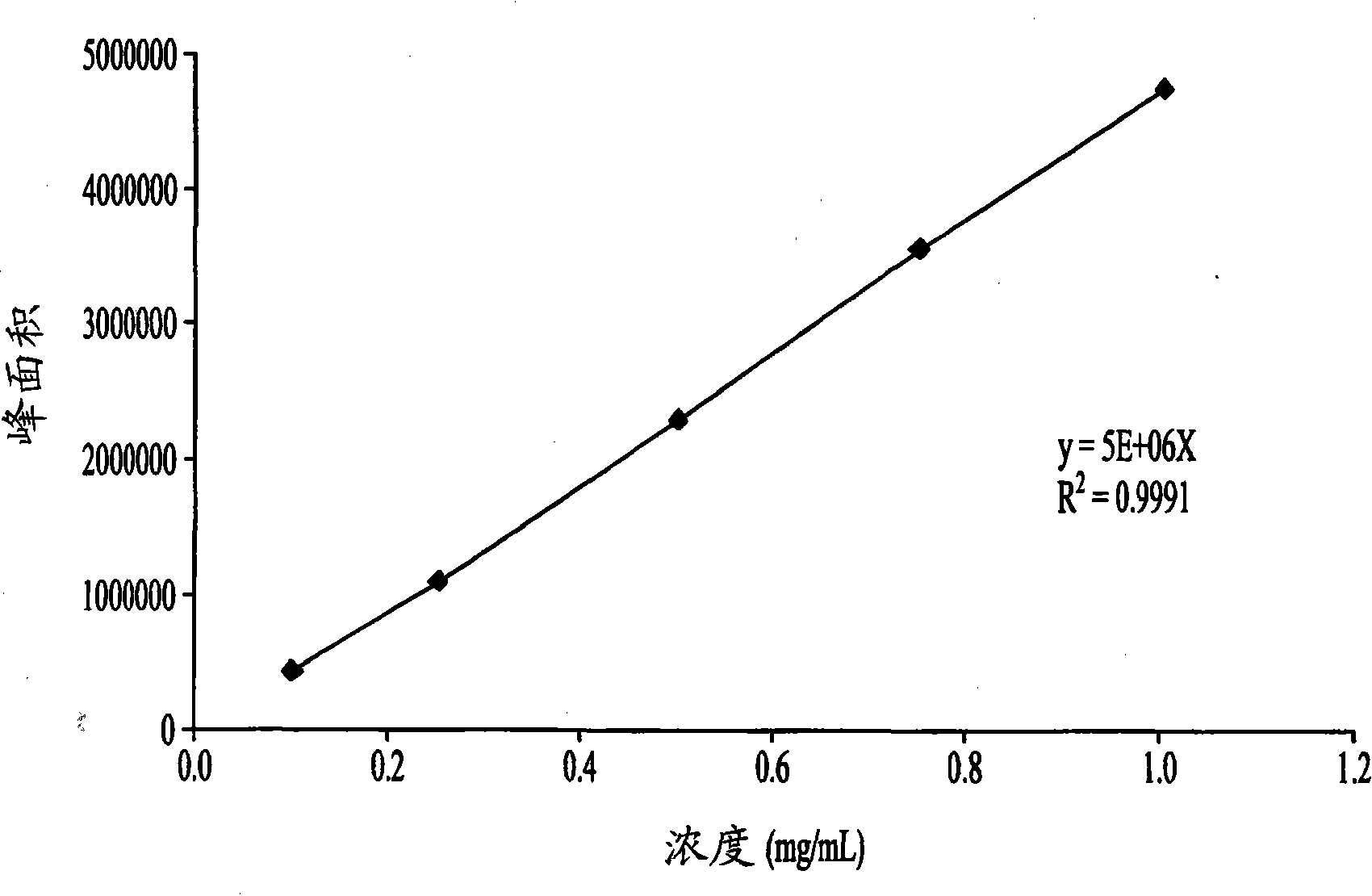
[0139] Example 1: HPLC method for picoplatin
[0140] condition:
[0141] column:
Luna 5u C18(2)250×4.6mm 00G-4252-E0
(Phenomenex)
Mobile phase A:
0.2% TFA in deionized water (v / v)
Mobile phase B:
Methanol HPLC grade
Flow rate:
1.0mL / min
Detection wavelength:
267nm
35℃
25℃
[0142] Time to go:
25 minutes
Sample diluent:
normal saline
[0143] Table I - Gradient
[0144]
Embodiment 2
[0145] Example 2: Determination of the solubility of picoplatin at various pH values.
[0146] The goals of this study were to determine the solubility of picoplatin in aqueous solutions and to measure the effect of pH on picoplatin solubility.
[0147] Table II - pH Buffers
[0148]
[0149]
[0150] step:
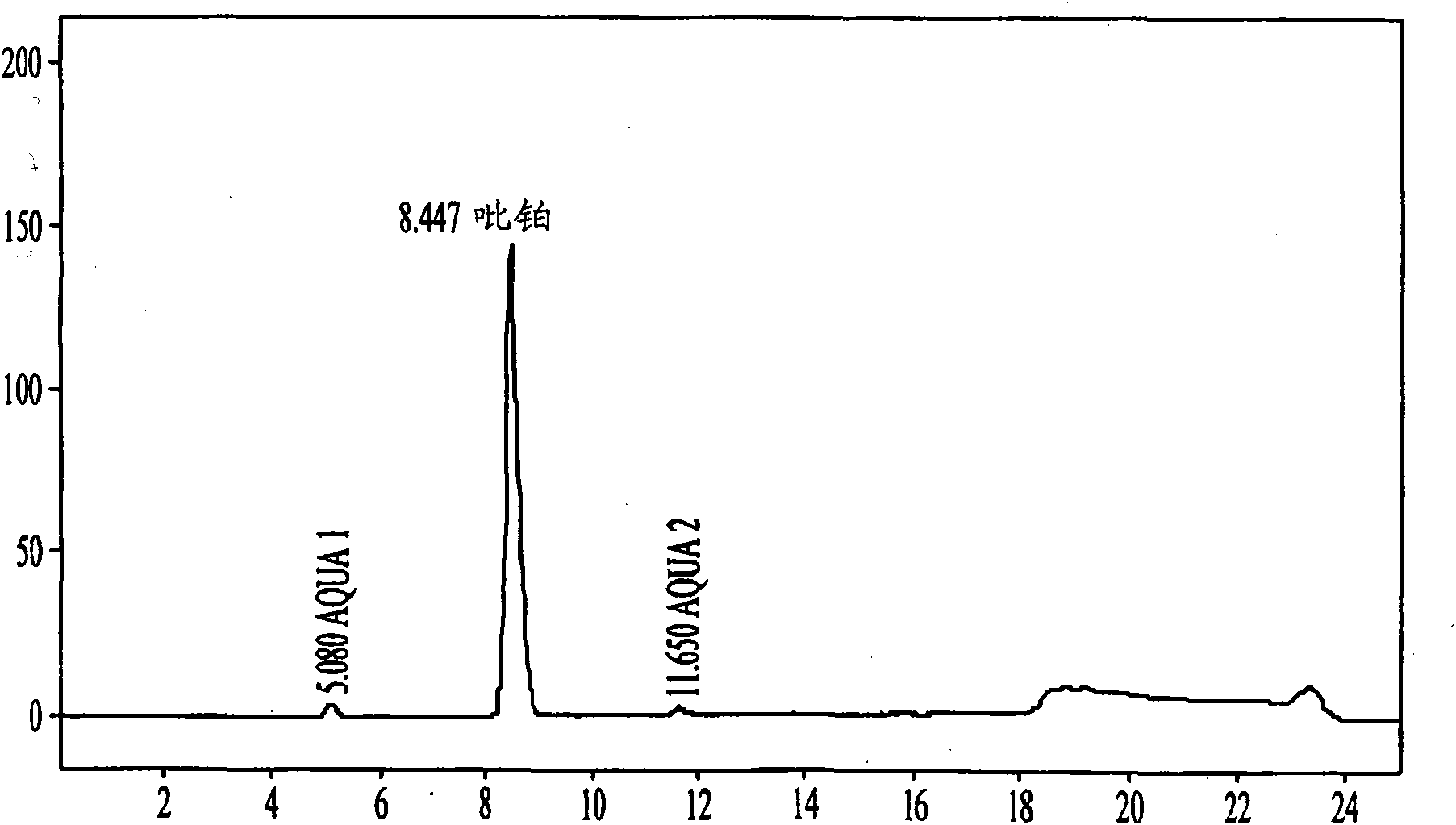
[0151] Picoplatin (10 mg) was weighed into 0.5 mL Eppendorf tubes, 10 tubes in total, and 250 μL of buffer or water was added to these picoplatins. The tubes were mixed for 1 minute. Measure the pH of each tube. The tubes were then placed on a shaker at 25°C in the dark for 16 hours and the pH was measured again. These solutions were centrifuged through 0.45 uM Spin-X filters, and 50 mg of each filtrate was transferred to individual HPLC tubes. 1.5 mL of 0.9% NaCl solution (physiological saline) was added to these HPLC tubes, followed by HPLC analysis immediately to determine the concentration of each sample.
[0152] Table III - pH of picoplatin in bu...
Embodiment 3
[0155] Example 3: Determination of the pH-stability profile of picoplatin.
[0156] The goals of this study were to determine the effect of pH on the stability of picoplatin in aqueous solution and to evaluate the overall stability of picoplatin in aqueous solution.
[0157] Table IV - pH Buffers
[0158]
[0159] step:
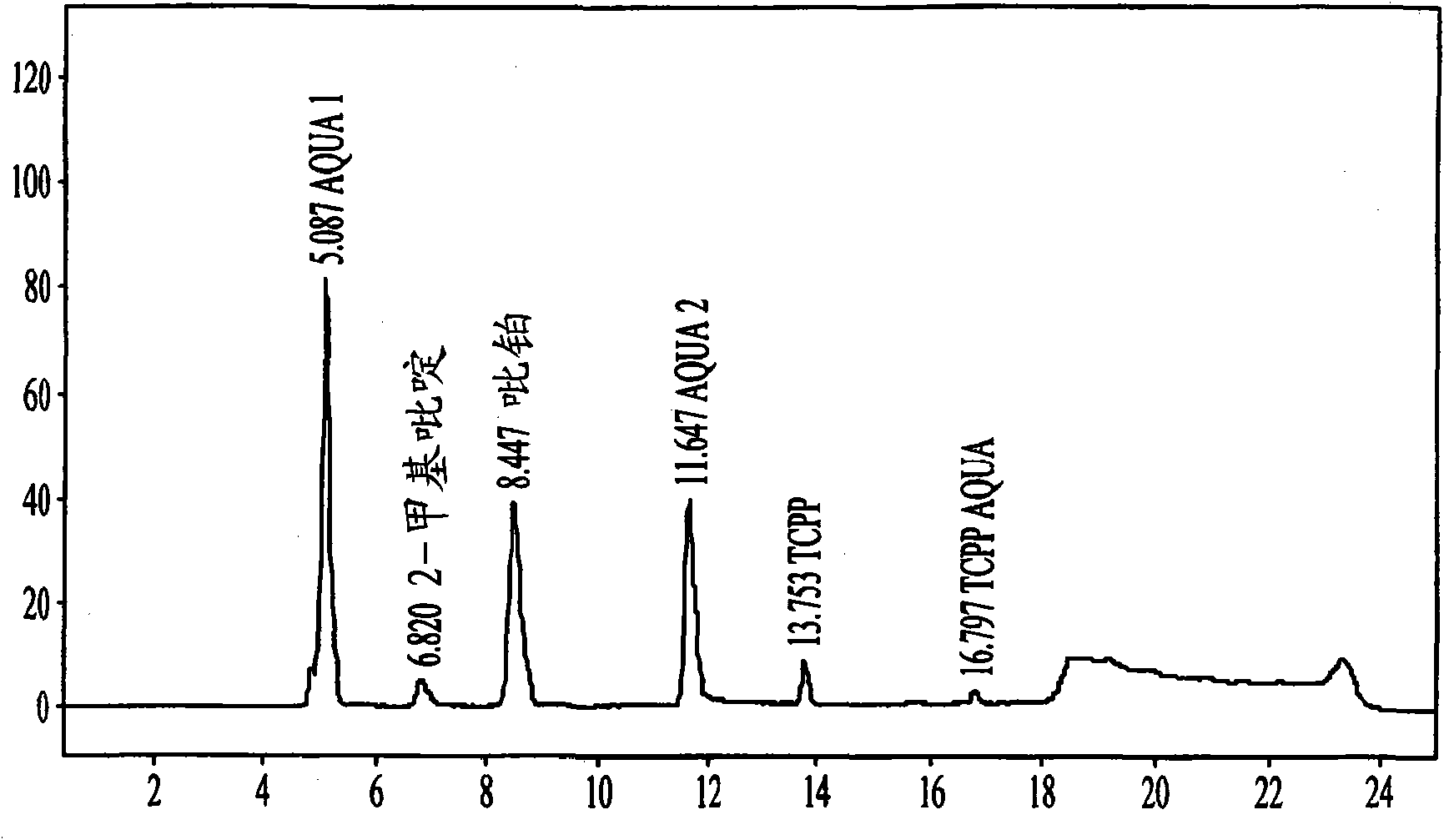
[0160]Picoplatin (10 mg + / - 0.1 mg) was weighed into a 5 mL volumetric flask, then saline was added to the 5 mL volume and the sample was mixed by inversion to dissolve all solids to obtain a 2 mg / mL stock solution. Then, 0.375 mL of the stock solution was added to 1.125 mL of the indicated pH buffer, deionized water, or saline in the HPLC tube and mixed by swirling for 10 seconds to obtain a 0.5 mg / mL test solution. Two tubes were made for each pH for checking.
[0161] Samples were then injected for HPLC analysis, one analysis per tube in the following order: pH 6, pH 5, pH 4, pH 3, pH 2, deionized water, saline.
[0162] Then, for each solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com