Method to treat small cell lung cancer
A technology for small cell lung cancer and therapy, applied in botany equipment and methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems that chemotherapy does not meet the requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0058] Example 1. Phase II Study
[0059] Phase II study of picoplatin monotherapy in patients with co-occurring SCLC with non-responsive SCLC, or SCLC that responded to first-line therapy but then progressed within 90 days of completion of first-line therapy, or progressed at 91-180 days Disease (as defined herein). Use picoplatin at 150mg / m 2 Doses administered intravenously every 21 days over a 1-2 hour period treated a cohort of 77 patients with measurable disease comprising 44 SCLC patients treated with first-line organoplatinum chemotherapy (cisplatin, carboplatin, or oxali Platinum) nonresponders and 27 SCLC relapsed within 90 days of stopping first-line therapy and 6 patients with progressive SCLC between 91 and 180 days old. Picoplatin is supplied as a sterile isotonic 0.5 mg / mL aqueous solution for IV infusion.
[0060] Patients received 1-10 cycles of picoplatin. The median number of dose cycles administered was 2 and the mean number of dose cycles was 3. Adver...
example 2
[0076] Example 2. Phase III study
[0077] introduce
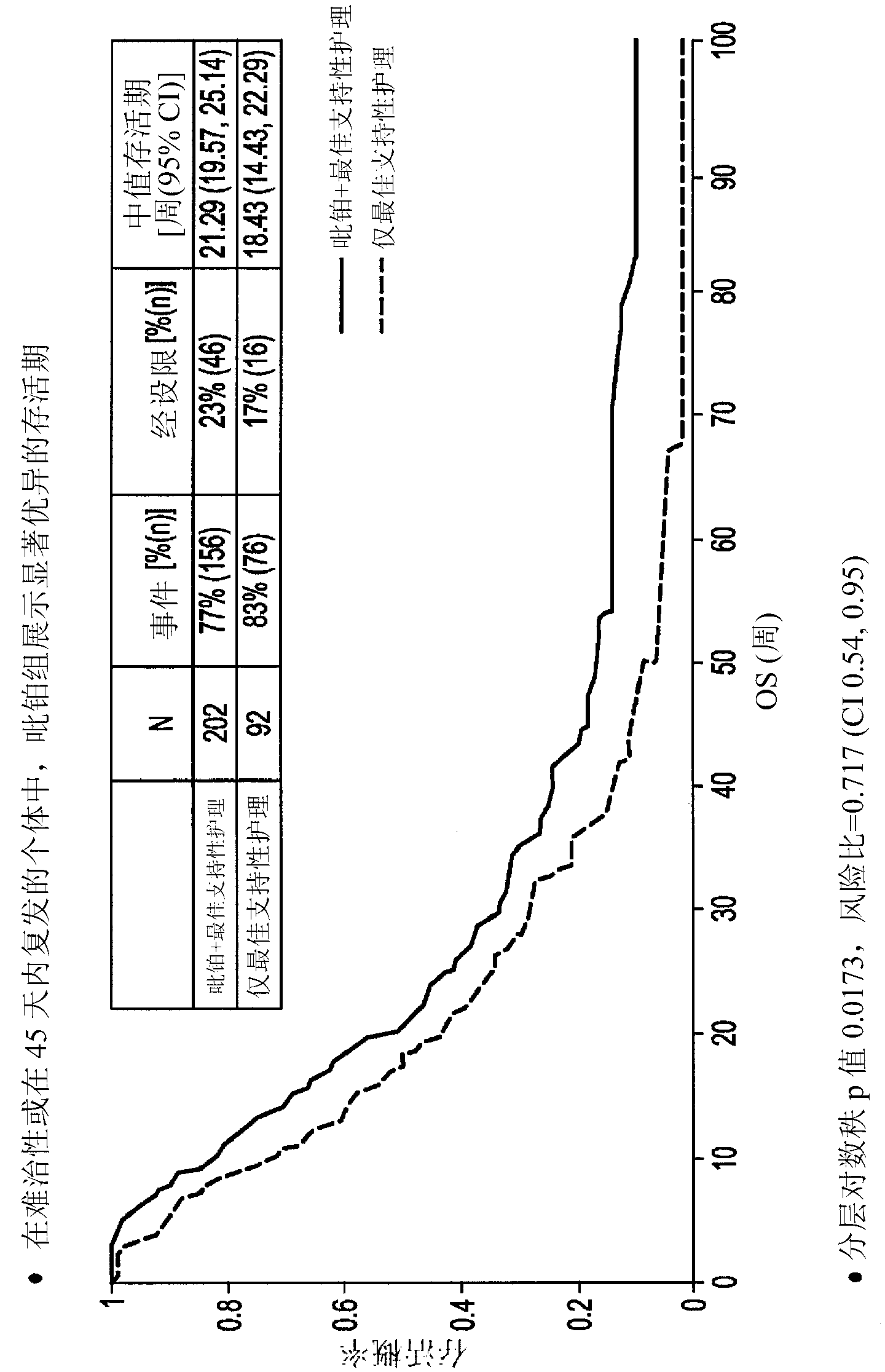
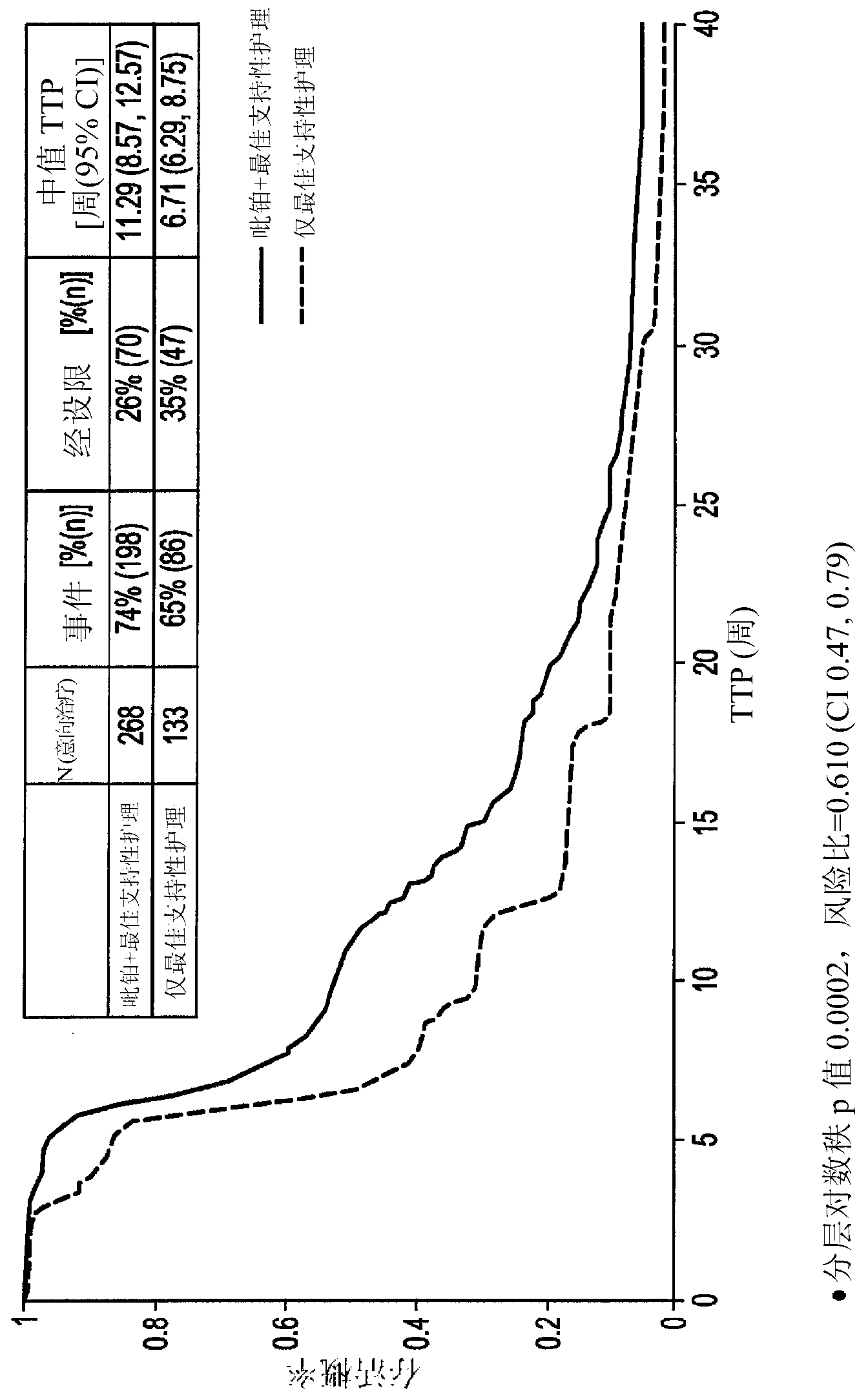
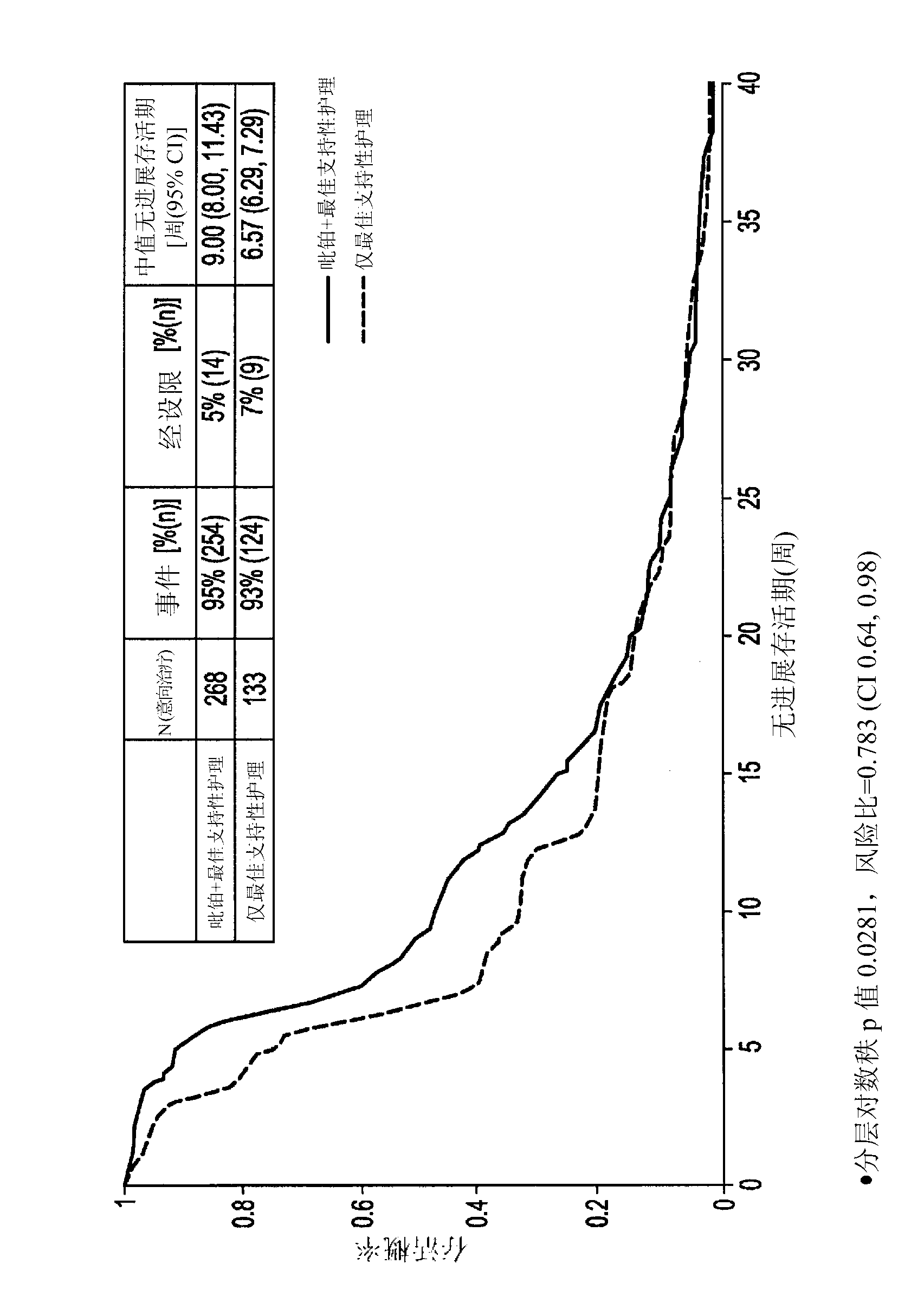
[0078] In non-responsive SCLC patients or responsive SCLC patients (including refractory and sensitive patients, as defined above) who show progressive disease within 180 days, conduct a phase III clinical study to illustrate the difference with best supportive care (BSC) only Median survival superiority compared with picoplatin monotherapy versus best supportive care.
[0079] Documentation about the treatment of the disease
[0080] Radiation therapy documentation for disease prior to first-line therapy must be available so that disease status at study baseline can be assessed for protocol eligibility and stratification purposes. The investigators determined eligibility by comparing chest x-rays or computed tomography (CT) scans obtained before, during, and after first-line chemotherapy. During screening, a baseline CT or magnetic resonance imaging (MRI) scan is performed for tumor evaluation.
[0081] The radiol...
example 3
[0463] Example 3. Treatment of SCLC brain metastases
[0464] Intracranial orthotopic SCLC model
[0465] Charles River Laboratories, Discovery and Imaging Services (formerly MIR Preclinical Services), Ann Arbor, MI in an Orthotopic Model of SCLC Brain Metastases (Ann Arbor, MI)) to evaluate picoplatin efficacy. will be 10 6 DMS114ACLC cells were implanted intracranially into 9-10 week old female athymic nude mice (outbred nu / nu). Treatment with vehicle or picoplatin (35 mg / kg) was initiated on day 18 with a dosing regimen of Q7DX4 at a mean tumor weight of 13 mg. Tumor mass and doubling time (Td) were assessed twice weekly using magnetic resonance imaging (MRI). use[ 18 F] Fluorothymidine positron emission tomography (FLT PET) as an indicator of tumor proliferation index, which is expressed in the form of standardized uptake value (SUV), and calculated as follows: SUV = (average tumor 18 F activity (μCi / g) x body weight (g)) ÷ decay-adjusted injected dose (μCi).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com