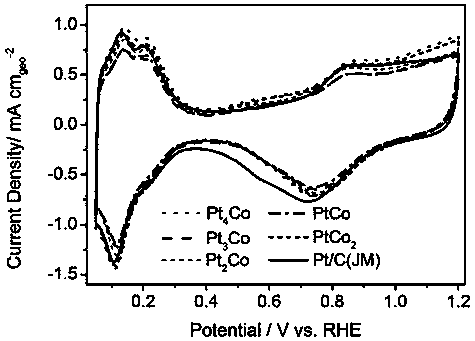
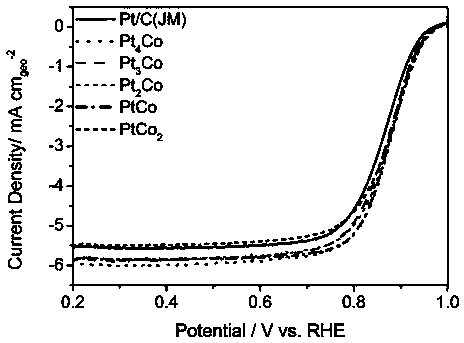
Preparation method of supported platinum-based alloy catalyst for low temperature fuel cell
A fuel cell, platinum-based alloy technology, applied in battery electrodes, nanotechnology for materials and surface science, circuits, etc., can solve complex operation of sodium borohydride reduction method, uneven distribution of catalyst particles, excessive alloy particle size to improve the catalytic activity of oxygen reduction, the preparation method is simple and effective, and the effect of reducing the amount of platinum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) H 2 PtCl 6 ·6H 2 O and CoCl 2 ·6H 2 O was dissolved in 2ml deionized water, and the atomic ratios of Pt to Co were 4:1, 3:1, 2:1, 1:1, 1:2. Stir to combine evenly.
[0029] (2) In ethylene glycol, add the carrier, stir for 10 minutes, and ultrasonically 30 minutes to make it evenly dispersed;
[0030] (3) Stir the dispersion at room temperature for 30 min, and feed N 2 Or Ar, forming an inert atmosphere of the reaction system;
[0031] (4) The reducing agent was added to the above dispersion liquid, followed by the mixed solution of Pt and Co precursors, and stirred at room temperature for 3 h. The total molar concentrations of Pt and Co elements in the system are respectively 3.6mmol / L, 3.75mmol / L, 3.9mmol / L, 4.2mmol / L and 4.95mmol / L, and the molar concentrations of the reducing agent are respectively the total molar concentrations of Pt and Co elements 18.5, 17.8, 17.4, 16.6, 14.9 times the concentration.
[0032] (5) After the reaction, separate by centr...
Embodiment 2
[0034] (1) The water-soluble Pt precursor K 2 PtCl 4and Ni precursor NiCl 2 ·6H 2 O (please give specific example) is dissolved in 2ml of deionized water, and the atomic ratio of Pt to Ni is 1:2. Stir to combine evenly.
[0035] (2) In ethylene glycol, add the carrier, stir for 10 minutes, and ultrasonically 30 minutes to make it evenly dispersed;
[0036] (3) Stir the dispersion at room temperature for 30 min, and feed N 2 Or Ar, forming an inert atmosphere of the reaction system;
[0037] (4) Add the reducing agent to the above dispersion liquid, then add the Pt and Ni precursor mixed solution, and stir at room temperature for 12h, so that the total concentration of Pt and Ni elements in the system is 6mmol / L, and the molar concentration of the reducing agent is Pt And 12.7 times the total molar concentration of Ni element.
[0038] (5) After the reaction, separate by centrifugation or filtration, wash with ethanol and deionized water for 5 times respectively, and add...
Embodiment 3
[0040] (1) Na 2 PtCl 6 ·6H 2 O and Ni(NO 3 ) 2 ·6H 2 O is dissolved (please give a specific example) in 2ml deionized water, and the atomic ratio of Pt to Ni is 1:2. Stir to combine evenly.
[0041] (2) In ethylene glycol, add the carrier, stir for 10 minutes, and ultrasonically 30 minutes to make it evenly dispersed;
[0042] (3) Stir the dispersion at room temperature for 30 min, and feed N 2 Or Ar, forming an inert atmosphere of the reaction system;
[0043] (4) Add the reducing agent to the above dispersion liquid, and then add the Pt and Ni precursor mixed solution, so that the total concentration of Pt and Ni elements in the system is 6mmol / L, and the molar concentration of the reducing agent is the total molar concentration of Pt and Ni elements 12.7 times. Stir at 80°C reaction temperature for 3h.
[0044] (5) After the reaction, separate by centrifugation or filtration, wash with ethanol and deionized water for 4 times respectively, and then vacuum dry at 60...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com