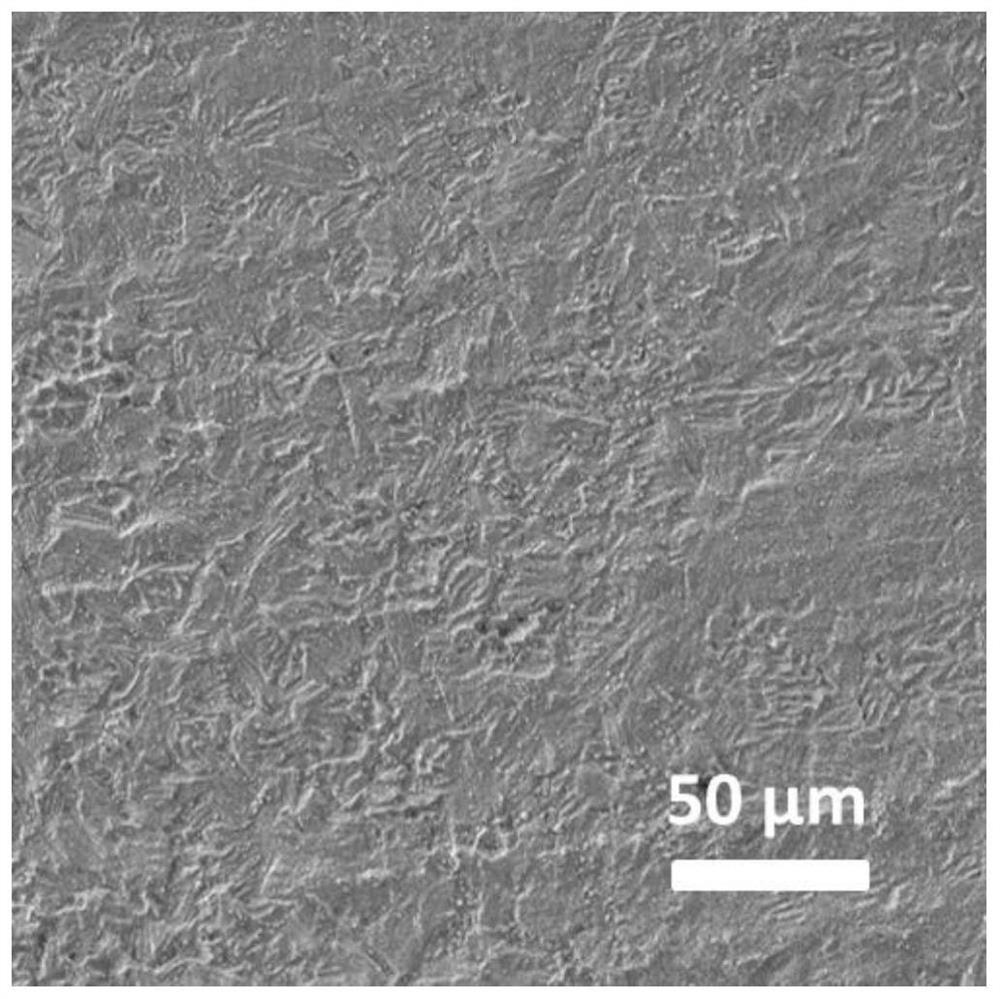
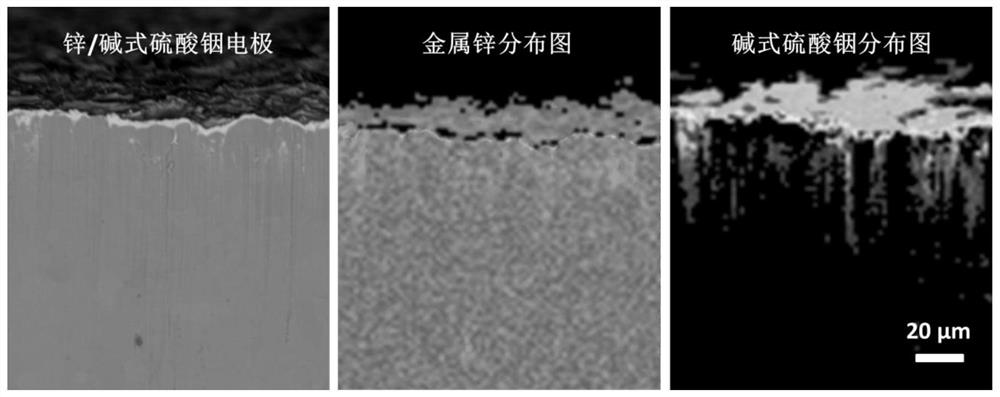
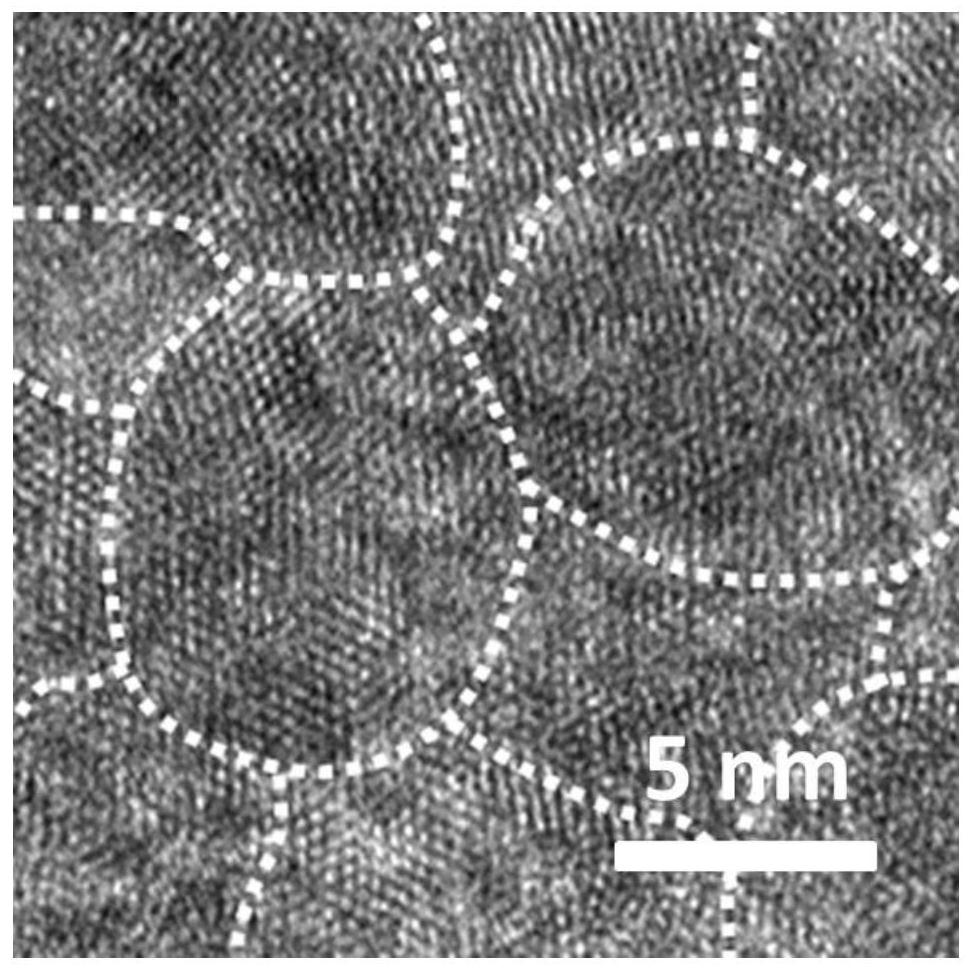
Long-cycle-life aqueous zinc secondary battery negative electrode and preparation and application thereof
A secondary battery and negative electrode technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of reducing the cycle life of zinc secondary batteries, side reaction dendrite growth, etc., to inhibit the growth of metal dendrites and improve the cycle. Life, the effect of optimizing the diffusion path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The zinc / basic indium sulfate electrode material was prepared by the following chemical displacement reaction method:
[0054] A. configuration reaction solution, the dilute hydrochloric acid that concentration is 0.1mol / L, the indium chloride ethanol solution that concentration is 0.1mol / L, the zinc sulfate aqueous solution that concentration is 3mol / L.
[0055] B. Pretreat the commercial polycrystalline metal zinc foil, ultrasonically clean it in 0.1mol / L dilute hydrochloric acid and deionized water for 2 minutes, and then immerse the pretreated zinc foil in the 0.1mol / L indium chloride ethanol solution of step A , stirred and reacted at normal temperature and pressure for 10 minutes, then taken out, washed and dried to obtain a zinc / indium precursor material.
[0056] C. Assembling the zinc / indium precursor material obtained in step B into a symmetrical battery, using the 3 mol / L zinc sulfate aqueous solution in step A as the electrolyte, and the glass fiber as the d...
Embodiment 2
[0067] The zinc / tin hydroxide electrode material was prepared by the following chemical displacement reaction method:
[0068] A. configuration reaction solution, the dilute hydrochloric acid that concentration is 0.1mol / L, the tin chloride ethanol solution that concentration is 0.1mol / L, the potassium hydroxide aqueous solution that concentration is 6mol / L.
[0069] B. Pretreat the commercial polycrystalline metal zinc foil, ultrasonically clean it in 0.1mol / L dilute hydrochloric acid and deionized water for 2 minutes, and then immerse the pretreated zinc foil in the 0.1mol / L tin chloride ethanol of step A The solution was stirred and reacted at normal temperature and pressure for 10 minutes, then taken out, washed and dried to obtain a zinc / tin precursor material.
[0070] C. Assembling the zinc / tin precursor material obtained in step B into a symmetrical battery, using the 6 mol / L potassium hydroxide aqueous solution in step A as the electrolyte, and the glass fiber as the ...
Embodiment 3
[0075] The zinc / alumina electrode material was prepared by the following chemical displacement reaction method:
[0076] A. configuration reaction solution, the dilute hydrochloric acid that concentration is 0.1mol / L, the aluminum chloride aqueous solution that concentration is 0.05mol / L, the zinc sulfate aqueous solution that concentration is 3mol / L.
[0077] B. Carry out pretreatment to commercial polycrystalline metal zinc foil, ultrasonically clean 2 minutes in 0.1mol / L dilute hydrochloric acid and deionized water, then immerse the pretreated zinc foil in the 0.05mol / L aluminum chloride aqueous solution of step A, After being stirred and reacted for 10 minutes at normal temperature and pressure, it was taken out, washed and dried to obtain a zinc / aluminum precursor material.
[0078] C. Assembling the zinc / aluminum precursor material obtained in step B into a symmetrical battery, using the 3 mol / L zinc sulfate aqueous solution in step A as the electrolyte, and the glass fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com