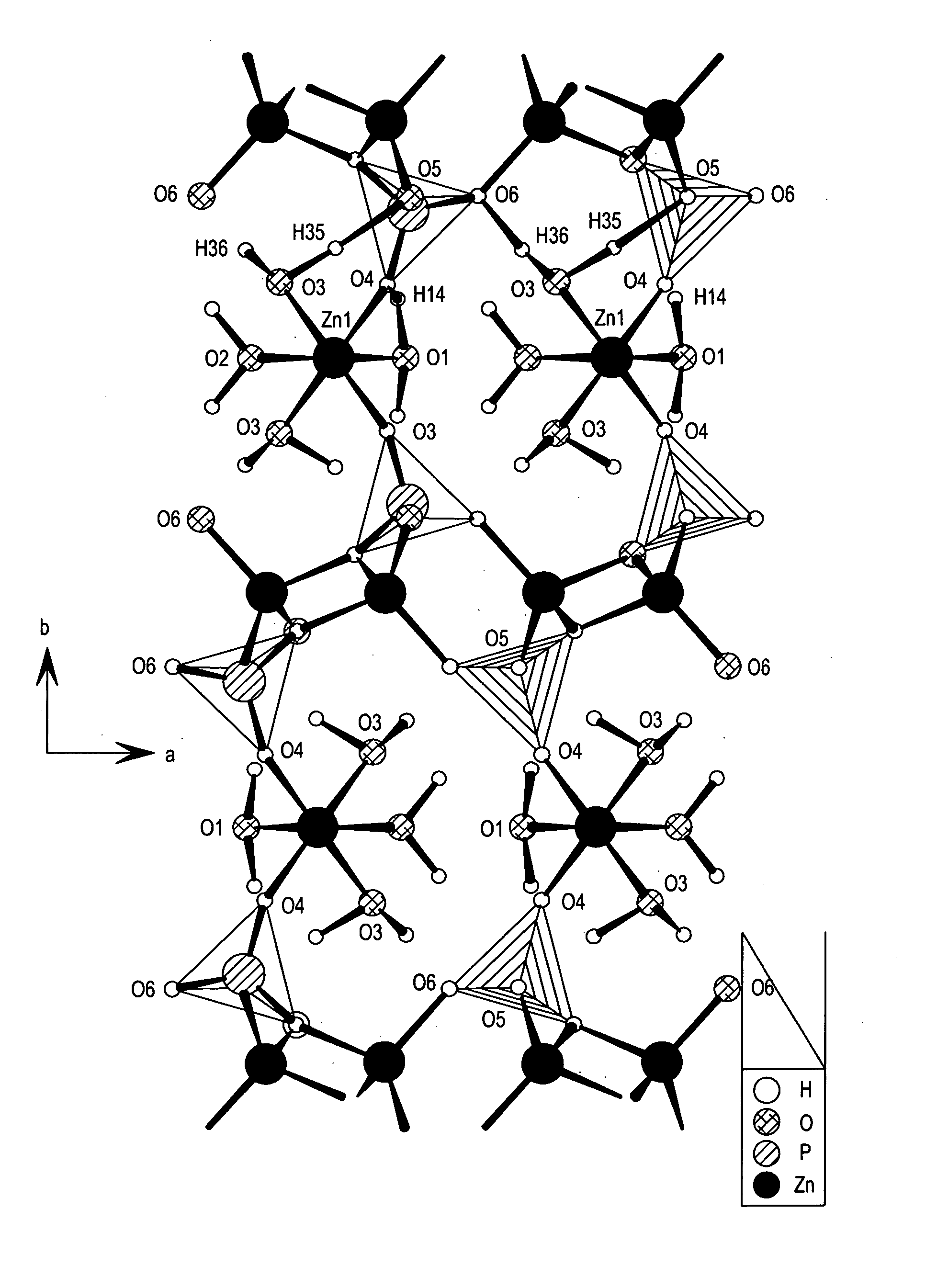
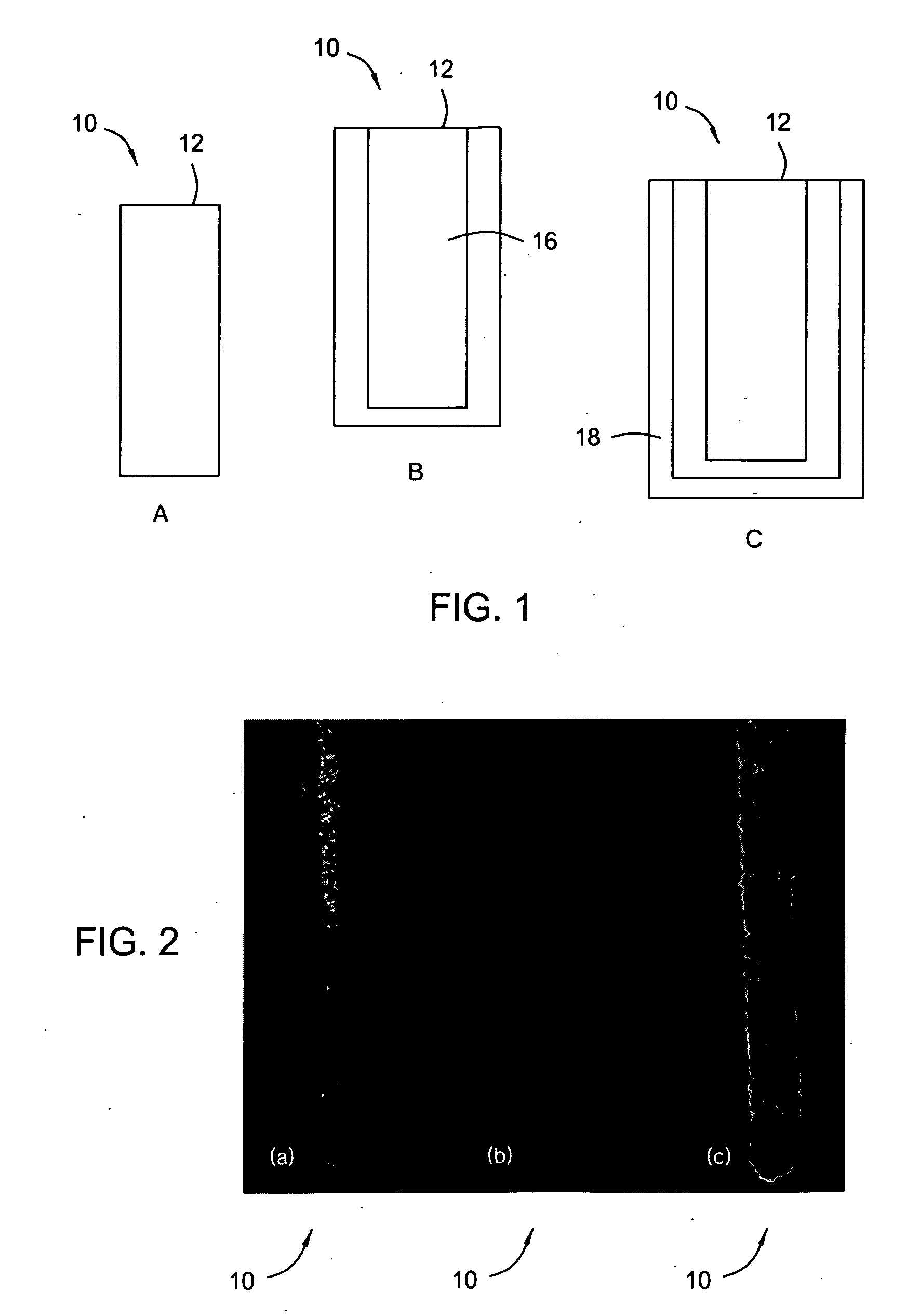
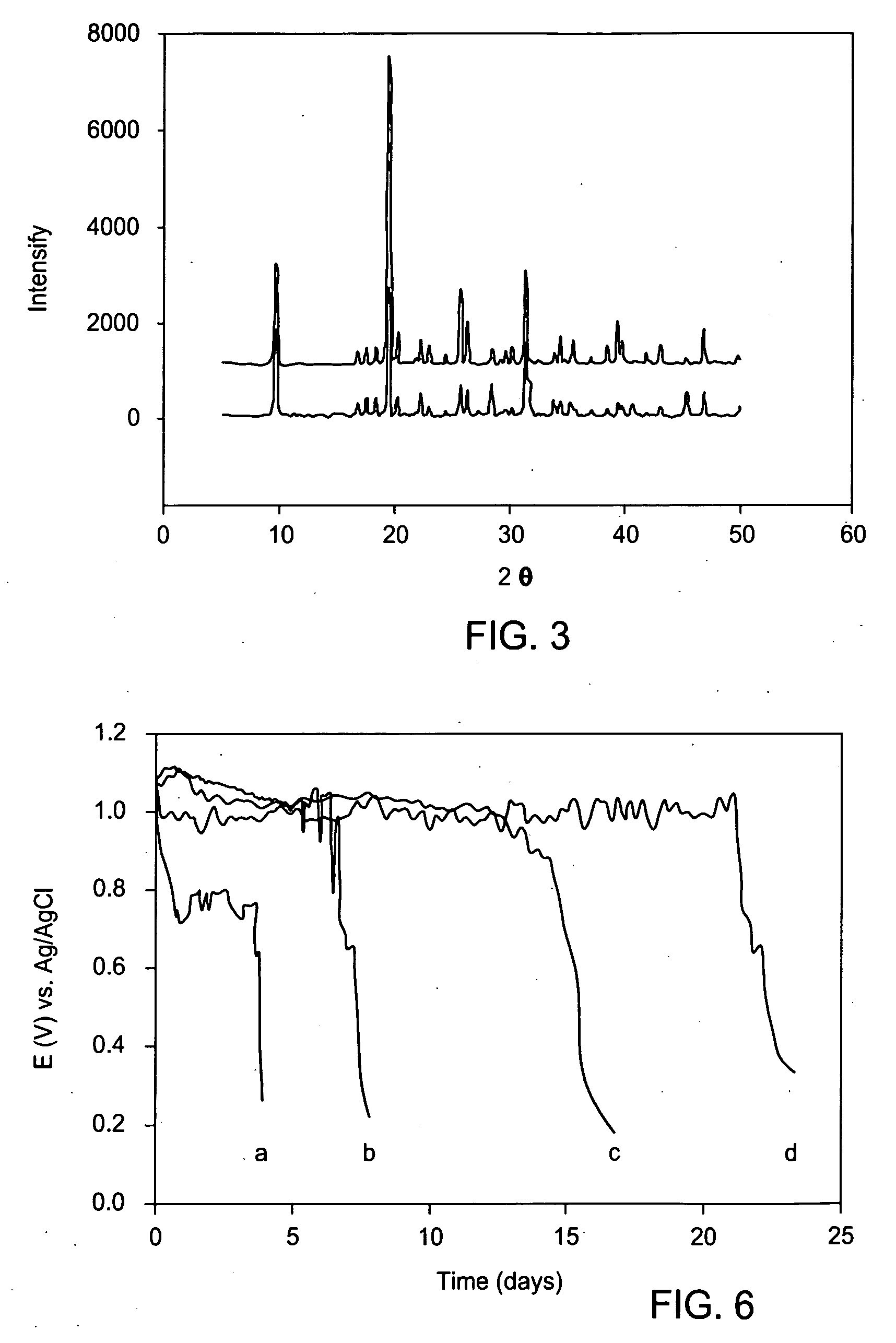
Reduction of the loss of zinc by its reaction with oxygen in galvanized steel and batteries
a technology of oxygen and zinc, applied in the direction of fuel and primary cells, cell components, manufacturing tools, etc., can solve the problem of reducing the coulombic efficiency of zinc utilization in the cathodic protection of steel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Glossary of Terms
[0031] Terms such as “a”, “an” and “the” are not intended to refer to only a singular entity, but include the general class of which a specific example may be used for illustration. The terminology herein is used to describe specific embodiments of the invention, but their usage does not delimit the invention, except as outlined in the claims.
[0032] Bath refers to a solution that directly contacts the substrate. The term “Bath” is not intended as a limitation of the manner of application of coating which generally can be applied to the substrate by various techniques, e.g., nonexclusive examples include immersion, dipping, spraying, placing the substrate into the bath, intermittent spraying, flow coating, and combined methods such as spraying-dipping-spraying, spraying-dipping, dipping-spraying or combinations thereof.
[0033] Electrolyte denotes a usually electronically insulating substance through which an electric current is carried through the motion of ions.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lengths | aaaaa | aaaaa |
| lengths | aaaaa | aaaaa |
| lengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com