Palladium-nickel-cobalt-sulfur composite nanotube array electrocatalyst grown on conductive substrate and preparation method and application of electrocatalyst
A nanotube array, conductive substrate technology, applied in the field of nanomaterials, can solve the problem of limited improvement of the catalytic performance of palladium electrolysis of water for hydrogen production, and achieve the effects of promoting electron transfer rate, good reproducibility, and improving diffusion speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A palladium-nickel-cobalt-sulfur composite nanotube array electrocatalyst grown on a conductive substrate is prepared by the following steps:
[0039] (1) Preparation of nickel-cobalt growth solution: 0.5 g nickel nitrate (0.034 mol / L), 1.0 g cobalt nitrate (0.043 mol / L), 0.3 g urea (0.062 mol / L) were dissolved in 80 mL aqueous solution, and stirred until Uniform;
[0040] The first kettle heat reaction: put the cleaned conductive substrate into a 20 mL reaction kettle, add 10 mL nickel-cobalt growth solution, immerse the conductive substrate, keep it in an oven at 150°C for 4 hours, and perform the first kettle heat reaction. After the reaction, cool to room temperature, wash with distilled water, and dry it in a 60°C oven to obtain a conductive substrate with nickel-cobalt compounds grown on it;
[0041] (2) Aqueous solution of sulfur-containing compounds: dissolve 2.5 g of sodium sulfide (0.352 mol / L) in 50 mL of distilled water, and stir until clear;
[0042] The ...
Embodiment 2
[0048] The palladium-nickel-cobalt-sulfur composite nanotube array electrocatalyst grown on the conductive substrate provided in this example was prepared by referring to the method of Example 1, the only difference being that 66.6 μL of concentrated HCl was added to the palladium source solution to make the palladium source solution acidic .
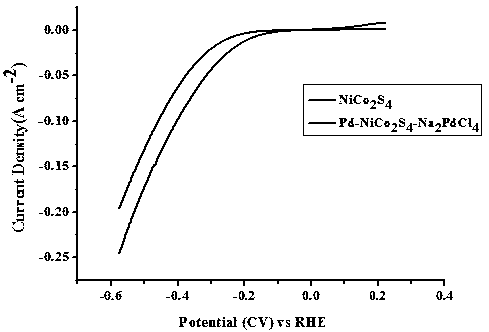
[0049] The palladium-nickel-cobalt-sulfur composite nanotube array electrocatalyst grown on the conductive substrate obtained in embodiment 2 is characterized by SEM, and the results are as follows: Figure 4 As shown; under the three-electrode system (same as Example 1), the palladium-nickel-cobalt-sulfur composite nanotube array electrocatalyst was tested for electrocatalytic water desorption hydrogen, and the results are as follows Figure 5 shown.
[0050] pass Figure 4 and figure 2 contrast, Figure 5 and image 3 The comparison shows that under different pH conditions, the microscopic morphology of the palladium-nickel-coba...
Embodiment 3
[0052] The palladium-nickel-cobalt-sulfur composite nanotube array electrocatalyst grown on the conductive substrate provided in this example was prepared with reference to the method of Example 2, the only difference being that when preparing the palladium source solution, 3.91 mg of sodium chloropalladate was replaced with 2.36 mg palladium chloride (1.33 mmol / L).
[0053] The palladium-nickel-cobalt-sulfur composite nanotube array electrocatalyst grown on the conductive substrate obtained in embodiment 3 is characterized by SEM, and the results are as follows: Image 6 As shown; under the three-electrode system (same as Example 1), the palladium-nickel-cobalt-sulfur composite nanotube array electrocatalyst was tested for electrocatalytic water desorption hydrogen, and the results are as follows Figure 7 shown.
[0054] pass Image 6 and Figure 4 contrast, Figure 7 and Figure 5 The comparison shows that the microscopic morphology of palladium-nickel-cobalt-sulfur co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com