Patents
Literature
592 results about "Cobalt compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
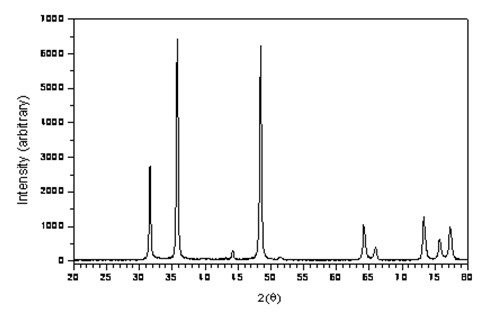
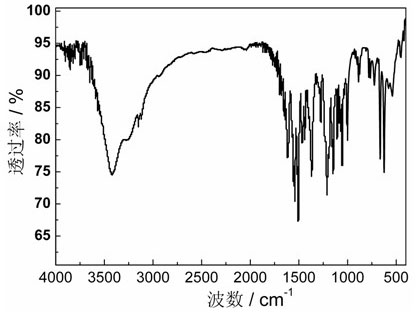
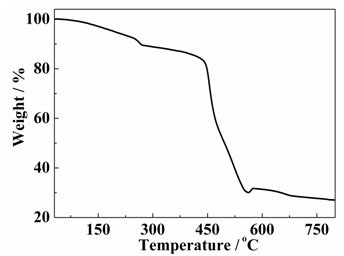
Cobalt: compounds information This section lists some binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and some other compounds of cobalt. For each compound, a formal oxidation number for cobalt is given, but the usefulness of this number is limited for p-block elements in particular.
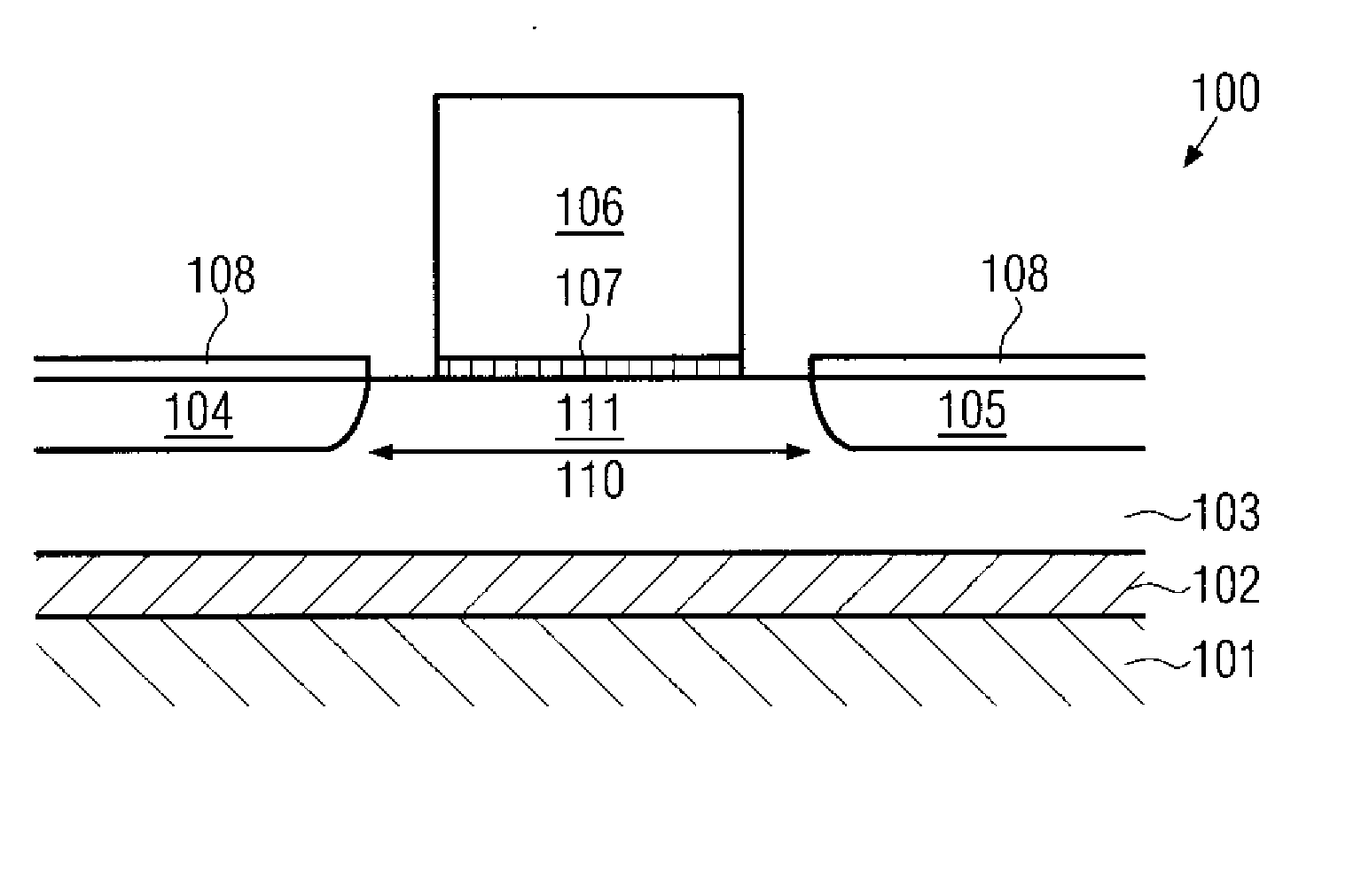
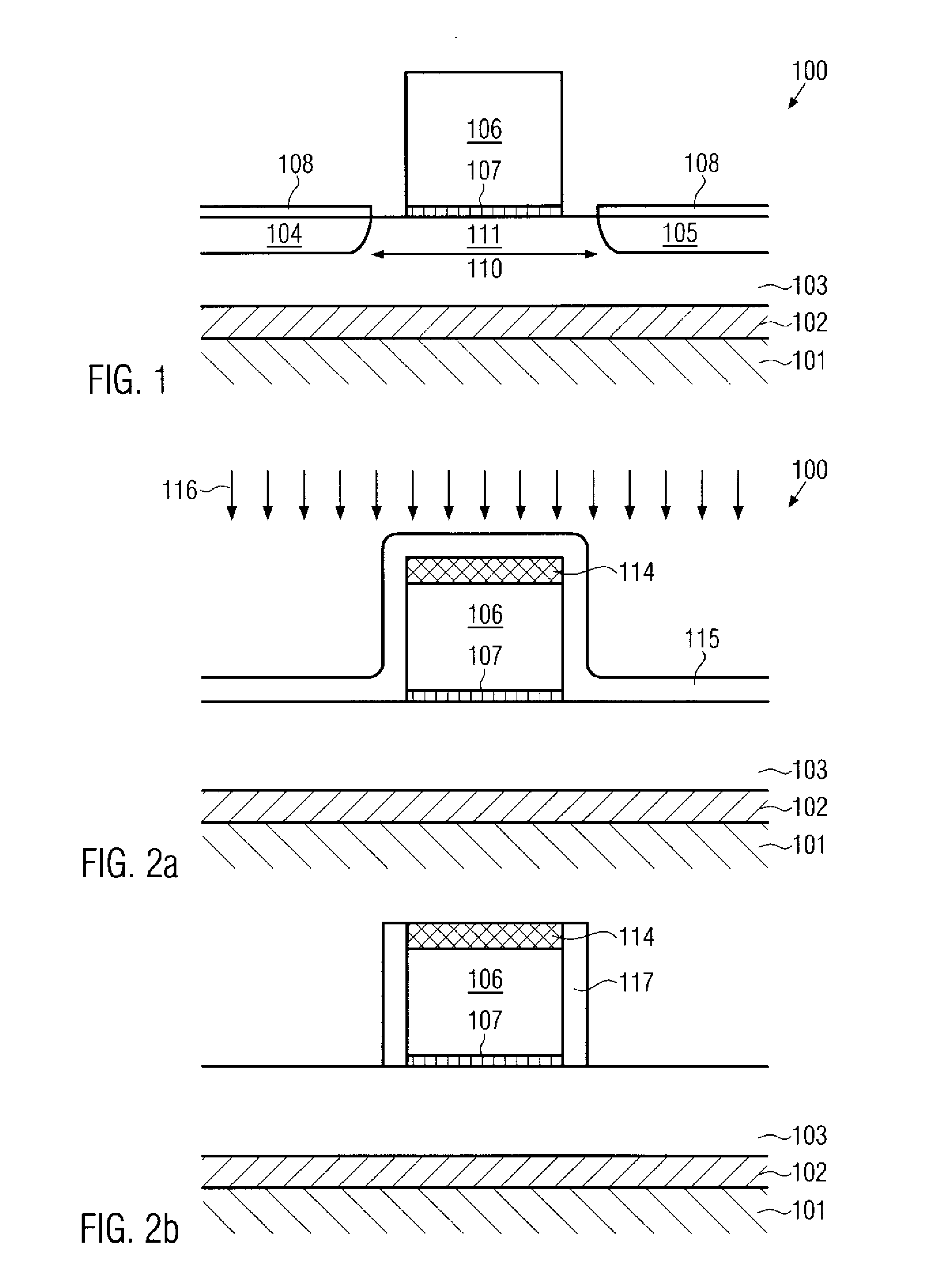
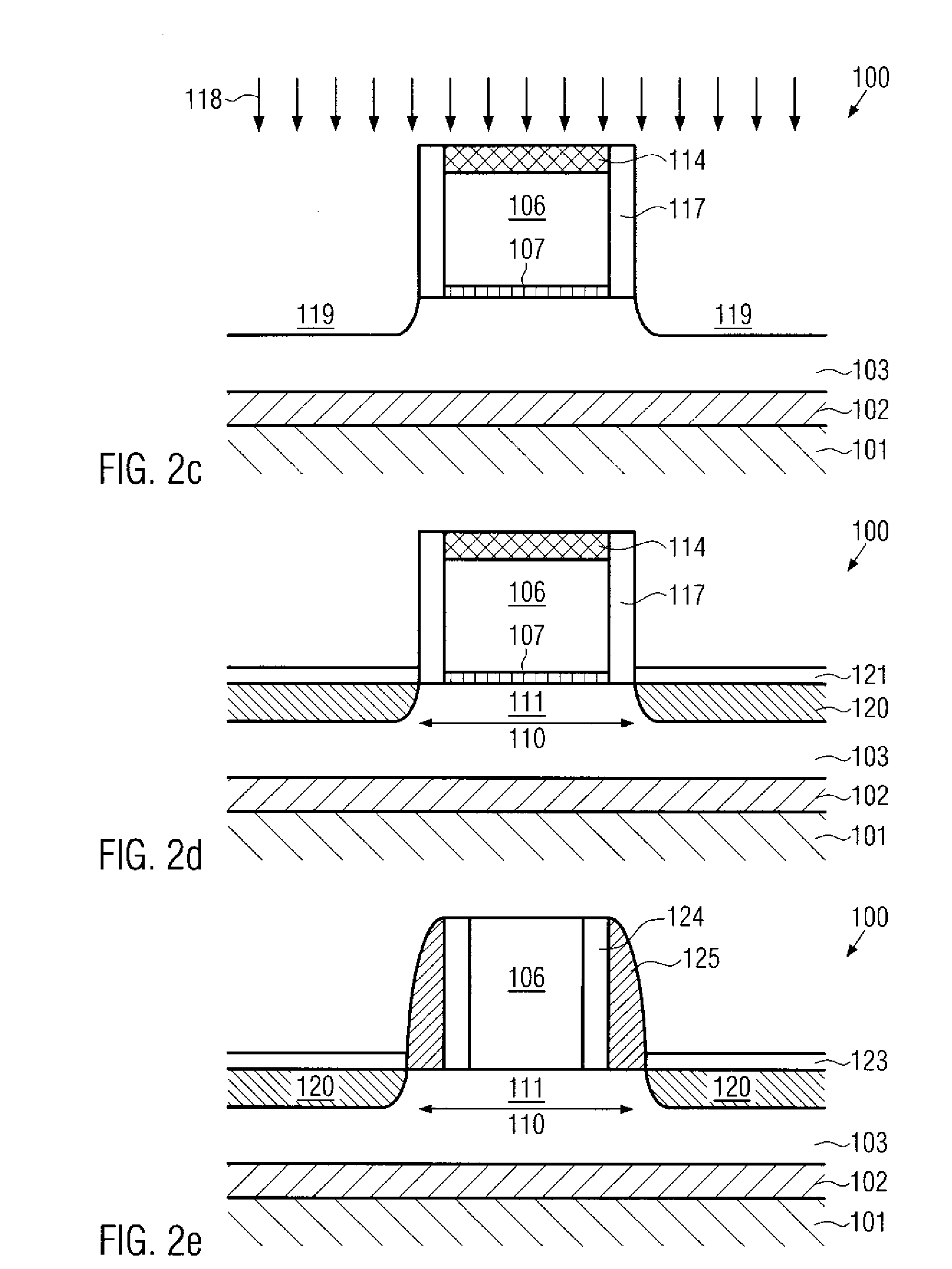
Formation of silicided surfaces for silicon/carbon source/drain regions
Formation of a silicide layer on the source / drain regions of a field effect transistor with a channel under tensile strain is disclosed. The strain is originated by the silicon / carbon source / drain regions which are grown by CVD deposition. In order to form the silicide layer, a silicon cap layer is deposited in situ by CVD. The silicon cap layer is then employed to form a silicide layer made of a silicon / cobalt compound. This method allows the formation of a silicide cobalt layer in silicon / carbon source / drain regions, which was until the present time not possible.
Owner:GLOBALFOUNDRIES INC
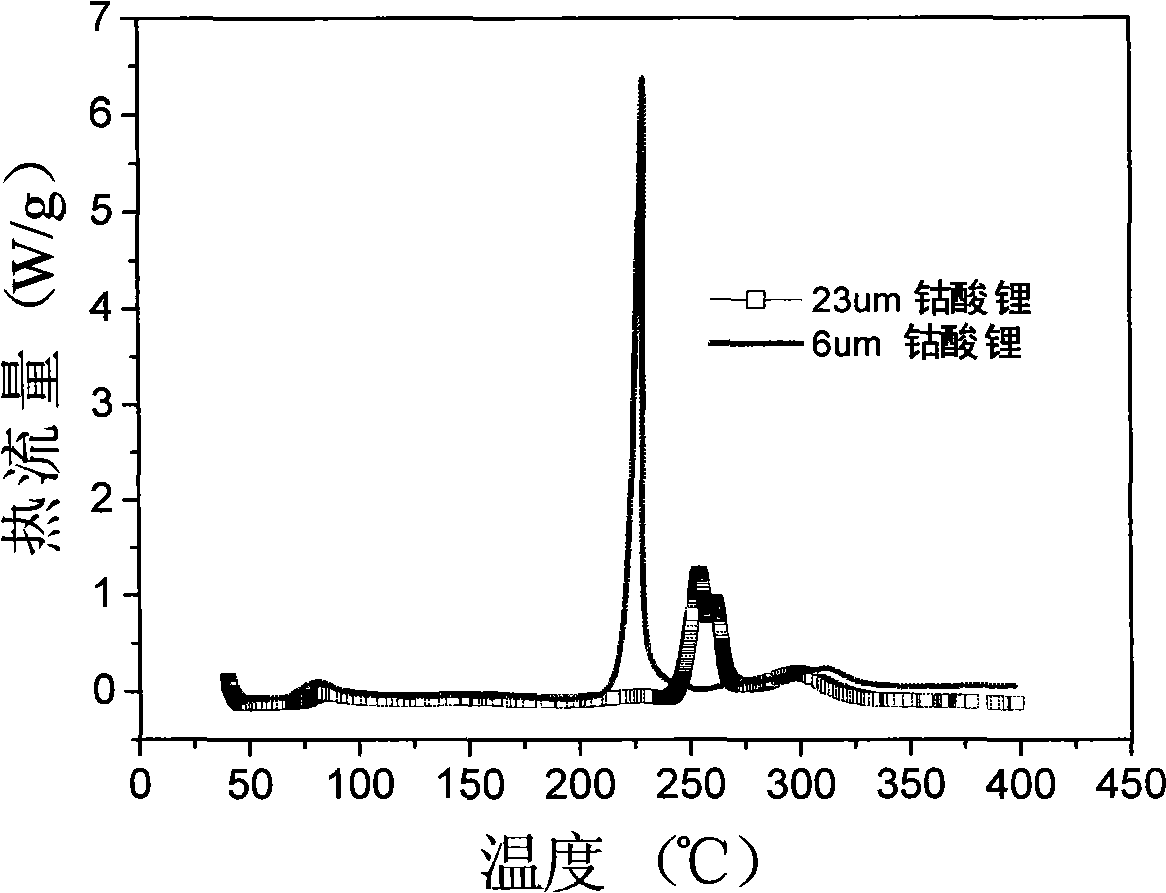
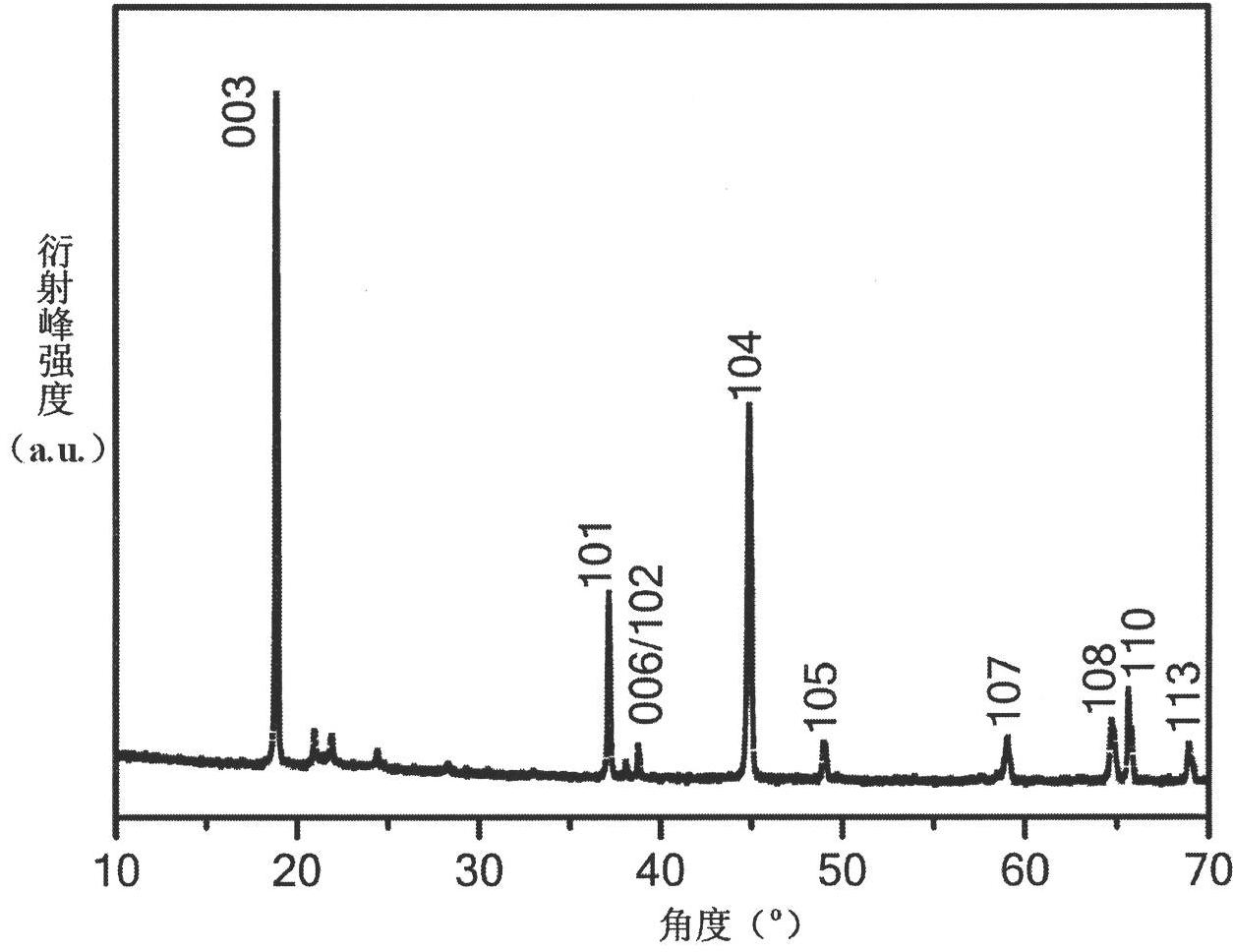
Super-size and high-density lithium cobalt oxide and method for preparing the same
ActiveCN101284681ALarge particle sizeHigh tap densityCell electrodesLithium compoundsHigh densityThermal impact
The invention relates to a high-density lithium cobalt oxide power material with a super-large grain diameter. The method comprises the steps of mixing a cobalt compound, a lithium compound or meanwhile a small amount of doping element compounds; sintering for 3 to 30 hours at the high temperature of 950 to 1,100 DEG C to form a block sintered product; crushing and grading the product to obtain the lithium cobalt oxide power material (molecular formula is LiaCo1-bMbO2), wherein, when b is not equal to 0, the middle diameter of the lithium cobalt oxide containing the doping elements is larger than or equal to 15 Mum, and the tap density is higher than or equal to 2.5g / cm<3>; when b is equal to 0, the middle diameter of the lithium cobalt oxide without the doping elements is larger than 20 Mum, and the tap density is higher than or equal to 2.6g / cm<3>. the 3.6V platform capacity rate of the material as the anode active substance for a lithium battery is higher than or equal to 75%; in the thermal impact test in a 150 DEG C thermotank, the lithium battery with the material is free from leakage and does not catch fire or explode for 60 minutes; the 1C5A specific capacity of the material in the battery is larger than or equal to 135mAh / g.
Owner:BEIJING EASPRING MATERIAL TECH CO LTD
Composite free layer for stabilizing magnetoresistive head
A magnetoresistive read head includes a spin valve having at least one free layer spaced apart from at least one pinned layer by a spacer. The free layer includes a cobalt compound as a thin film including at least one of Co—X, CoFe—X and CoNi—X, where X is an element from the lanthanoid family (a 4-f element). The content of Co is higher than 80 percent, and the content of the lanthanoid element is less than 10 percent. The film may comprise the entire free layer, or be positioned adjacent to one or more conventional free layer films. The pinned layer is a conventional single layer, or a synthetic multi-layered structure having a spacer between sub-layers. Because the spin valve structure has a high exchange stiffness and damping factor, spin transfer effect is reduced and a high-speed dynamic response is provided.
Owner:TDK CORPARATION
Articles derived from compositions containing modified polybutylene terephthalate (PBT) random copolymers derived from polyethylene terephthalate (PET)
Compositions of matter including articles derived from (a) from 5 to 99.99 wt % of a modified polybutylene terephthalate random copolymer that (1) is derived from polyethylene terephthalate and (2) contains a at least one residue derived from polyethylene terephthalate selected from the group consisting of antimony, germanium, diethylene glycol groups, isophthalic acid groups, cis isomer of cyclohexane dimethanol, trans isomer of cyclohexane dimethanol, sodium benzoate, alkali salts, napthalane dicarboxylic acids, 1,3-propane diols, cobalt, cobalt-containing compounds, and combinations thereof, and (b) from 0.01 to 95 wt. % of a member selected from the group consisting of (1) fillers, (2) a carboxy reactive component, (3) polyethyelene terephthalate, (4) a component including a polycarbonate and an impact modifier. The articles may be derived from various conversion processes, e.g., injection molding processes, extrusion processes, thermoforming processes, melt-blown process.
Owner:SHPP GLOBAL TECH BV
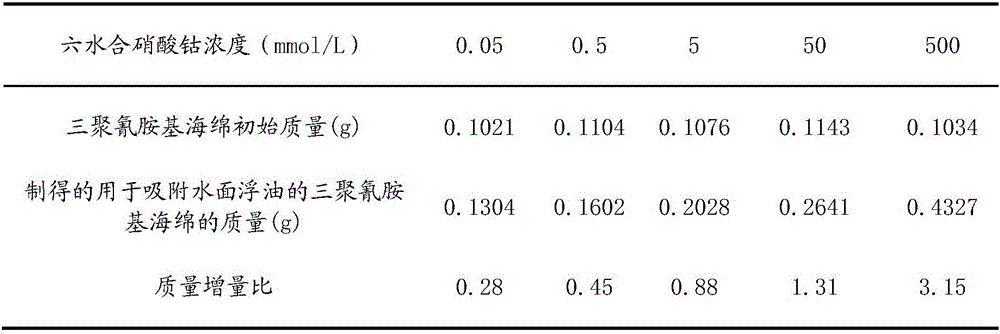
Melamine-based sponge for absorbing oil slick and preparation method
InactiveCN105949498AEasy to prepareMild reaction conditionsFatty/oily/floating substances removal devicesOther chemical processesAlcoholRoom temperature
The invention relates to melamine-based sponge for absorbing oil slick and a preparation method thereof. The preparation method includes the following steps of pretreatment: putting melamine-based sponge in absolute ethyl alcohol and deionized water for ultrasonic cleaning prior to drying; roughening: putting the pretreated melamine-based sponge in cobalt compound water solution, adding oxidant solution into cobalt compound water solution with stirring at room temperature, and after reaction, taking out and drying the melamine-based sponge; hydrophobic modification: soaking the roughened melamine-based sponge into hydrophobic modified reagent with stirring; after soaking, taking out the soaked melamine-based sponge prior to drying to obtain the melamine-based sponge for absorbing oil slick. The melamine-based sponge for absorbing oil slick is simple preparation method, mild in reaction condition, easily available in raw material, low in cost, high in oil absorption rate and good in reusability.
Owner:BEIJING FORESTRY UNIVERSITY
Preparation method of high-capacity layered lithium-rich manganese-based oxide
ActiveCN102354741AImprove charge and discharge efficiencyImprove cycle performanceCell electrodesManganeseNickel compounds
The invention relates to a preparation method of a high-capacity layered lithium-rich manganese-based oxide, and the method is characterized by comprising the following steps: a) fully mixing a nickel-containing compound, a manganese-containing compound I and a cobalt-containing compound with a solvent so as to get a solution or suspension; b) adding a precipitator into the solution or suspension obtained in the step a), and filtering to obtain a precipitate; c) drying the precipitate obtained in the step b) so as to get a precursor; d) uniformly mixing the precursor obtained in the step c), a manganese-containing compound II, a doping element M-containing compound and a lithium-containing compound in proportion so as to get mixed powder; and e) performing heat treatment on the mixed powder obtained in the step d) so as to get an ultrahigh-capacity manganese series lithium transition metal compound oxide with an expression of Li(Li0.2Mn0.8-delta-alpha-beta-Ni alpha Co beta M delta)O2. Compared with the prior art, the preparation method provided by the invention has the advantages of high utilization rate of raw materials and low production cost, and the prepared lithium-rich manganese-based oxide has the advantages of excellent charge-discharge performance and excellent cycle performance.
Owner:宁波富理电池材料科技有限公司
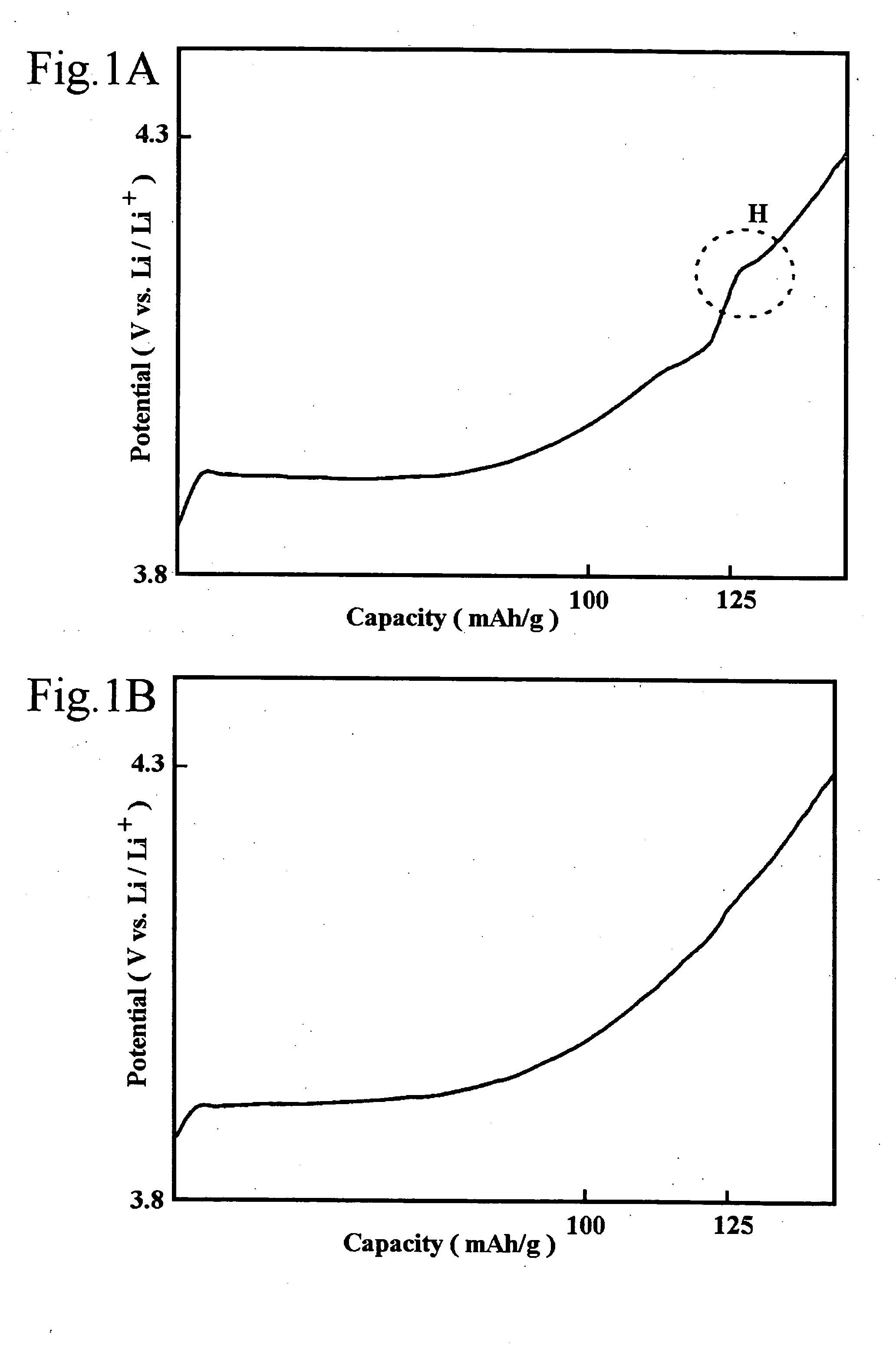
Non-aqueous electrolyte secondary battery and method of manufacturing the same
ActiveUS20050069774A1Excellent characteristicsImprove performanceElectrode thermal treatmentNon-aqueous electrolyte accumulatorsDischarge efficiencyCoprecipitation
A positive electrode used in the non-aqueous electrolyte secondary battery of the present invention includes a hexagonal system lithium-containing cobalt composite oxide represented by the general expression ┌LiCo1-XMXO2 (M=Zr, Mg, Al)┘ obtained by synthesizing a lithium compound as a lithium source with a cobalt compound as a cobalt source to which 0.01 mol % or more and 1.0 mol % or less of zirconium is added and magnesium and / or aluminum is added through coprecipitation, as the positive electrode active material, whereby the thermal stability, load performance and charging / discharging cycle performance characteristics of the non-aqueous electrolyte secondary battery are improved without lowering its capacity and charging / discharging efficiency.
Owner:PANASONIC ENERGY CO LTD
High-voltage charge type nonaqueous electrolyte secondary cell
ActiveUS20090181311A1Improve cycle performanceInhibit reduction in cycle propertyOrganic electrolyte cellsLi-accumulatorsNickel compoundsSolvent
The preservation performance of a nonaqueous electrolyte secondary cell charged to high potential is improved while the initial capacity and the cycle property of the cell are also improved. The nonaqueous electrolyte secondary cell includes: a positive electrode having lithium phosphate and a positive electrode active material containing lithium cobalt compound oxide and lithium manganese nickel compound oxide having a layer structure, the lithium cobalt compound oxide having at least zirconium and magnesium added in LiCoO2; a negative electrode having a negative electrode active material; and a nonaqueous electrolyte having a nonaqueous solvent and an electrolytic salt. The potential of the positive electrode is more than 4.3 V and 5.1 V or less based on lithium. The nonaqueous electrolyte contains vinylene carbonate as the nonaqueous solvent and, as the electrolytic salt, at least one of lithium bis(pentafluoroethane sulfonyl)imide and lithium bis(trifluoromethane sulfonyl)imide at 0.1 M or more and 0.5 M or less. The nonaqueous electrolyte contains 1,3-dioxane.
Owner:PANASONIC ENERGY CO LTD
Process for preparing alcohols from olefins by hydroformylation and hydrogenation
InactiveUS20060129004A1Downtime costEasy to preparePreparation by oxo-reaction and reductionOrganic compound preparationCobalt saltOxygen
A process for preparing aliphatic alcohols that includes cobalt-catalyzed hydroformylation of olefins, treatment of a hydroformylation mixture with oxygen-containing gases in the presence of acidic, aqueous cobalt(II) salt solutions, separation of a mixture into an aqueous phase comprising cobalt salts and an organic phase comprising the aliphatic aldehydes, and hydrogenation of an aldehyde-containing organic phase wherein the organic phase and treatment with an adsorbent to separate off cobalt compounds prior to hydrogenation.
Owner:EVONIK DEGUSSA GMBH
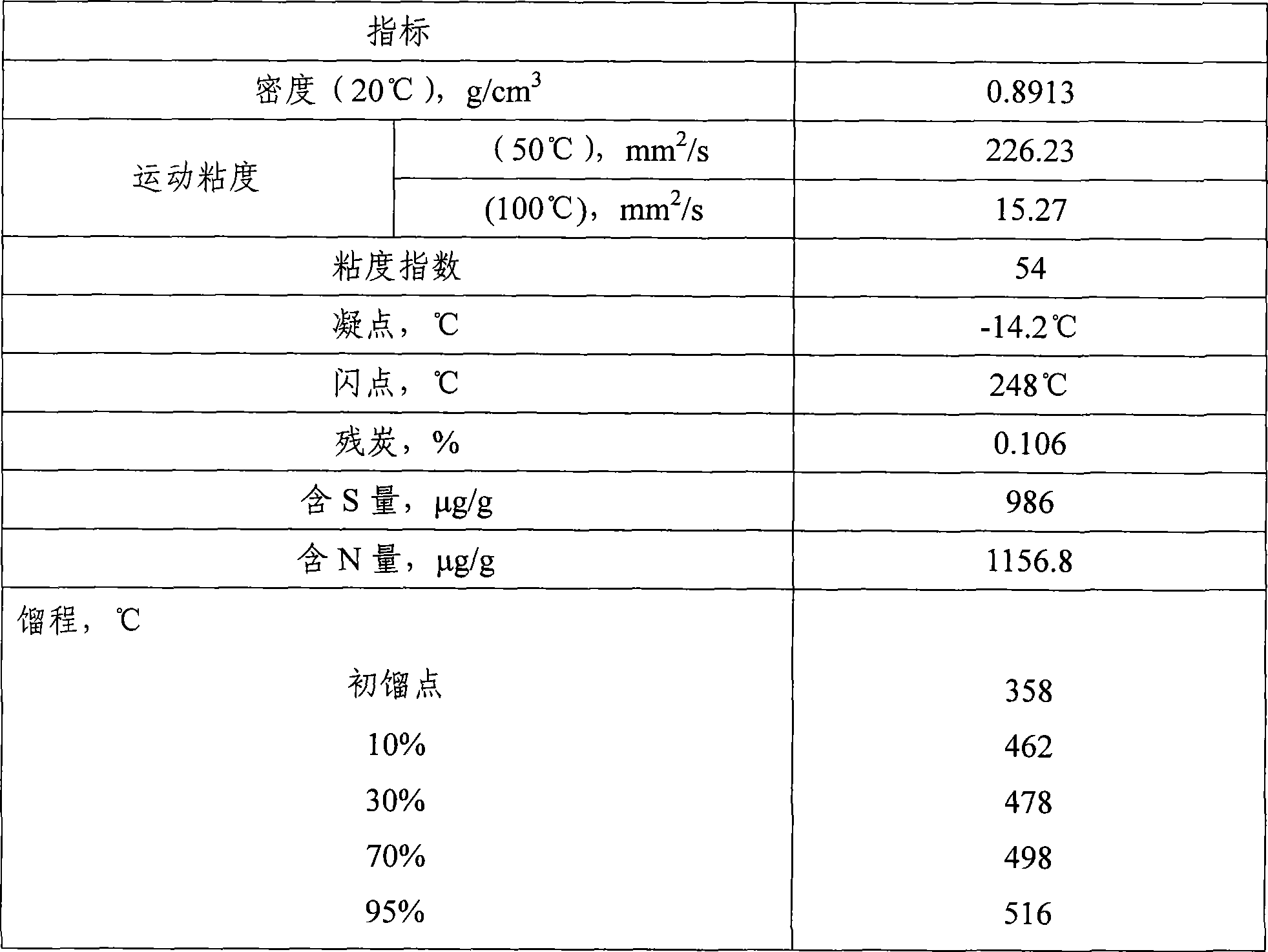
Teeth spherical heavy oil hydrotreating catalyst and preparation method thereof
ActiveCN101497044AAchieve technical effectLarge specific surface areaMetal/metal-oxides/metal-hydroxide catalystsHydrocarbon oils treatmentHydrodesulfurizationReaction temperature
The invention discloses a dentiform sphere shaped heavy oil hydrotreated catalyst and a preparation method thereof. The catalyst comprises an active composition, an addition agent and a dentiform sphere shaped aluminum oxide carrier, wherein the active composition comprises compounds of cobalt and nickel, and a compound of molybdenum or / and tungsten; the addition agent comprises a compound of phosphor, silicon, boron or halogen or compounds of any two compositions of the phosphor, the silicon, the boron and the halogen; and compositions according to gross weight of the dentiform sphere shaped heavy oil hydrotreated catalyst comprise: 2 to 6 percent of the cobalt compound, 3 to 10 percent of the nickel compound, 0 to 26 percent of the molybdenum compound, 0 to 8 percent of the tungsten compound, and 0.5 to 2 percent of the addition agent. The catalyst has the characteristics of high specific surface area, large pore volume, high strength, proper surface acid content, small bed pressure drop, low reaction temperature and high activity of hydrodesulfurization and denitrification. The preparation method comprises preparation of a precursor substance of the aluminum oxide carrier, preparation of the dentiform sphere shaped aluminum oxide carrier, preparation of a catalyst steeping fluid and preparation of the catalyst. The preparation process can be mastered and realized simply and easily.
Owner:BEIJING GAOXIN LIHUA TECH CO LTD
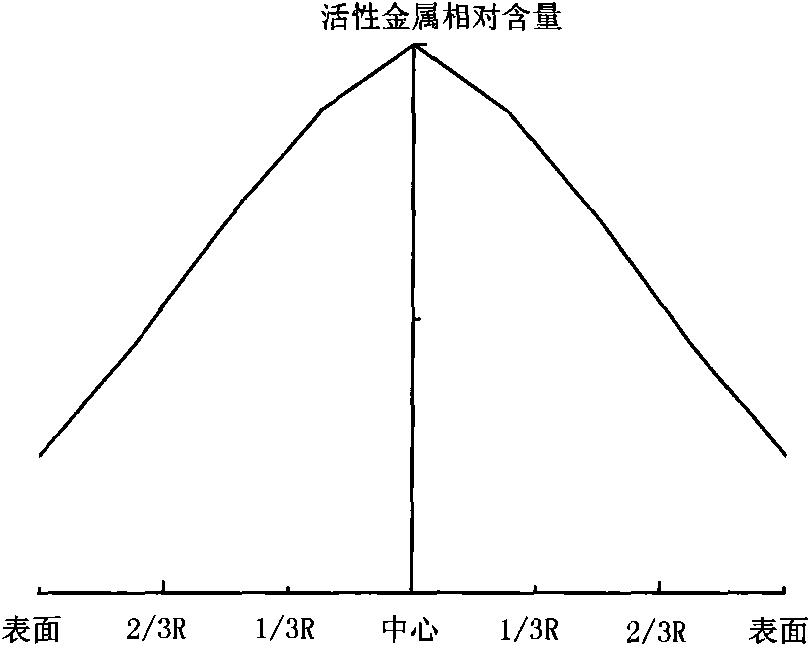
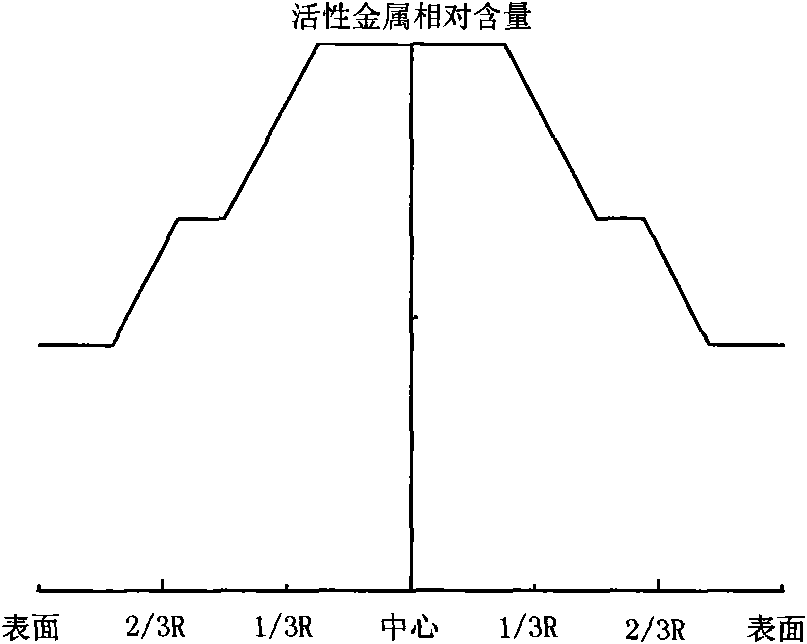
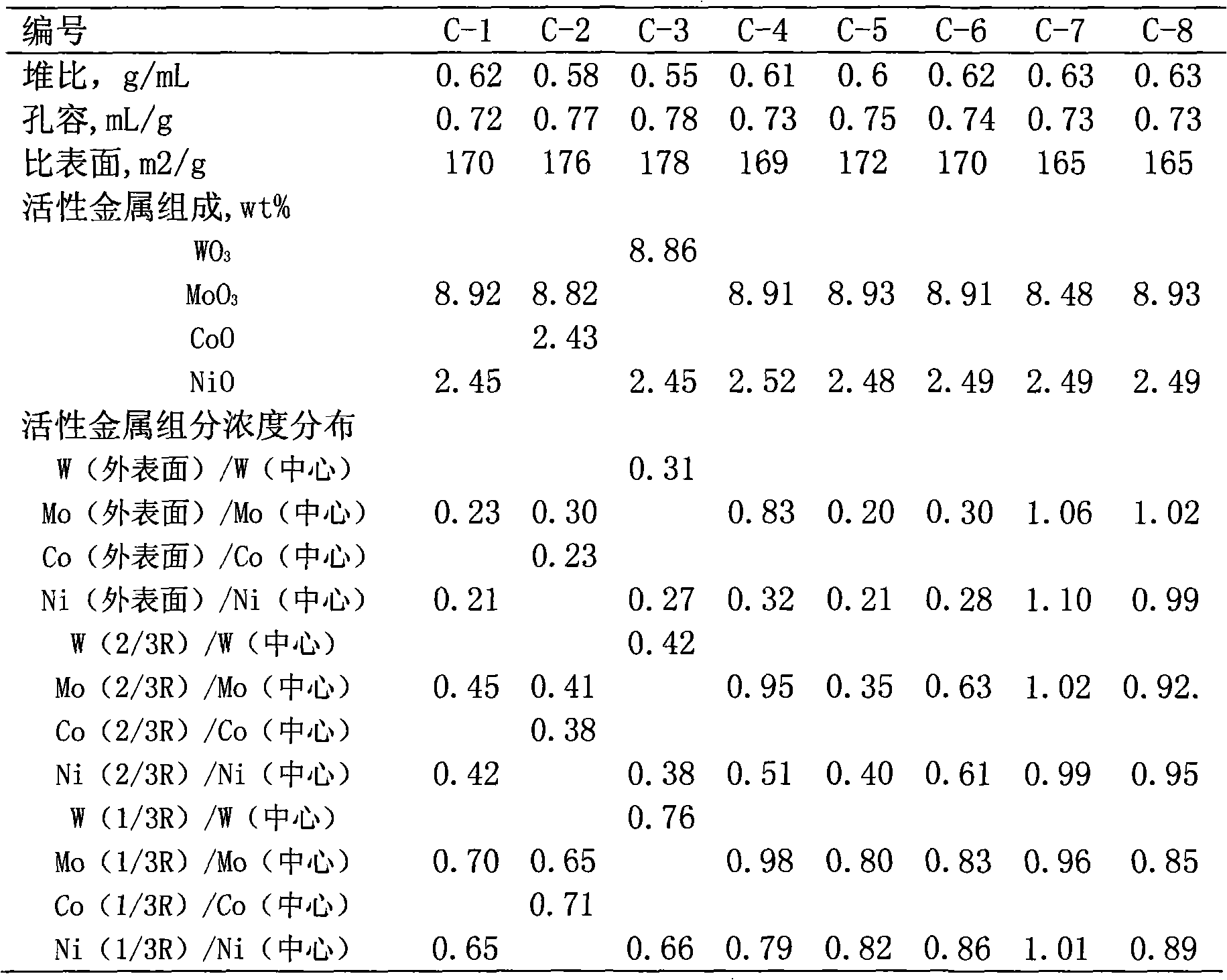
Hydrogenation catalyst with gradient-decreasing-distributed active metal constituent concentration and preparation method thereof
ActiveCN101927196AGood demetallizationGood charcoal removalCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsSal ammoniacAmmonia
The invention relates to a hydrogenation catalyst with gradient-decreasing-distributed active metal constituent concentration and a preparation method thereof. The preparation method comprises the following steps: by using Al2O3 or Al2O3 containing SiO2, TiO2 and ZrO2 as the carrier, mixing molybdenum and / or tungsten compounds and / or nickel and / or cobalt compounds with deionized water or ammonia water to obtain a metal dipping solution; preparing a thicker metal dipping solution dipping carrier, and gradually adding deionized water or ammonia water to dilute the metal dipping solution saturated spray carrier, or preparing metal dipping solutions with at least two different concentrations; and drying at 80-150 DEG C for 1-8 hours, and roasting in the air at 300-650 DEG C for 2-6 hours. The catalyst contains 1.0-10.0 wt% of molybdenum and / or tungsten oxides and / or 0.2-5.0 wt% of cobalt and / or nickel oxides. The hydrogenation catalyst has favorable activities of demetallization, carbon residue removal, devulcanization and denitrification, has the advantages of high stability, simple preparation and low cost, and is used for hydrogenating heavy-weight oil.
Owner:PETROCHINA CO LTD
Regenerable heterogeneous Fenton catalyst, and preparation method and application thereof
ActiveCN105478155AImprove stabilityGood regeneration performanceMolecular sieve catalystsWater treatment compoundsPhenolPollution
The invention specifically relates to a regenerable heterogeneous Fenton catalyst, and a preparation method and application thereof, which belongs to the technical field of industrial catalysts. The catalyst provided by the invention uses commercial zeolite as a carrier and is prepared through the following steps: surface modification of the carrier with a high polymer; loading of an active component Fe or Co; and roasting so as to form an iron or cobalt compound-valence oxide on the surface of zeolite. The catalyst shows high-efficiency degradability to industrial intractable organic waste water containing phenols, dyes or the like, and the degradation rate of organic matters in waste water is up to more than 90% at room temperature (less than 30 DEG C) under an almost neutral condition (wherein a pH value is in a range of 6 to 8); and the catalyst has good regeneration performance. According to the method, the heterogeneous Fenton catalyst is used for oxidation removal of organic matters like phenols and dyes in waste water, so cost is low and no secondary pollution is produced; and a process for treating waste water with the Fenton catalyst is a promising organic waste water treatment process.
Owner:FUDAN UNIV
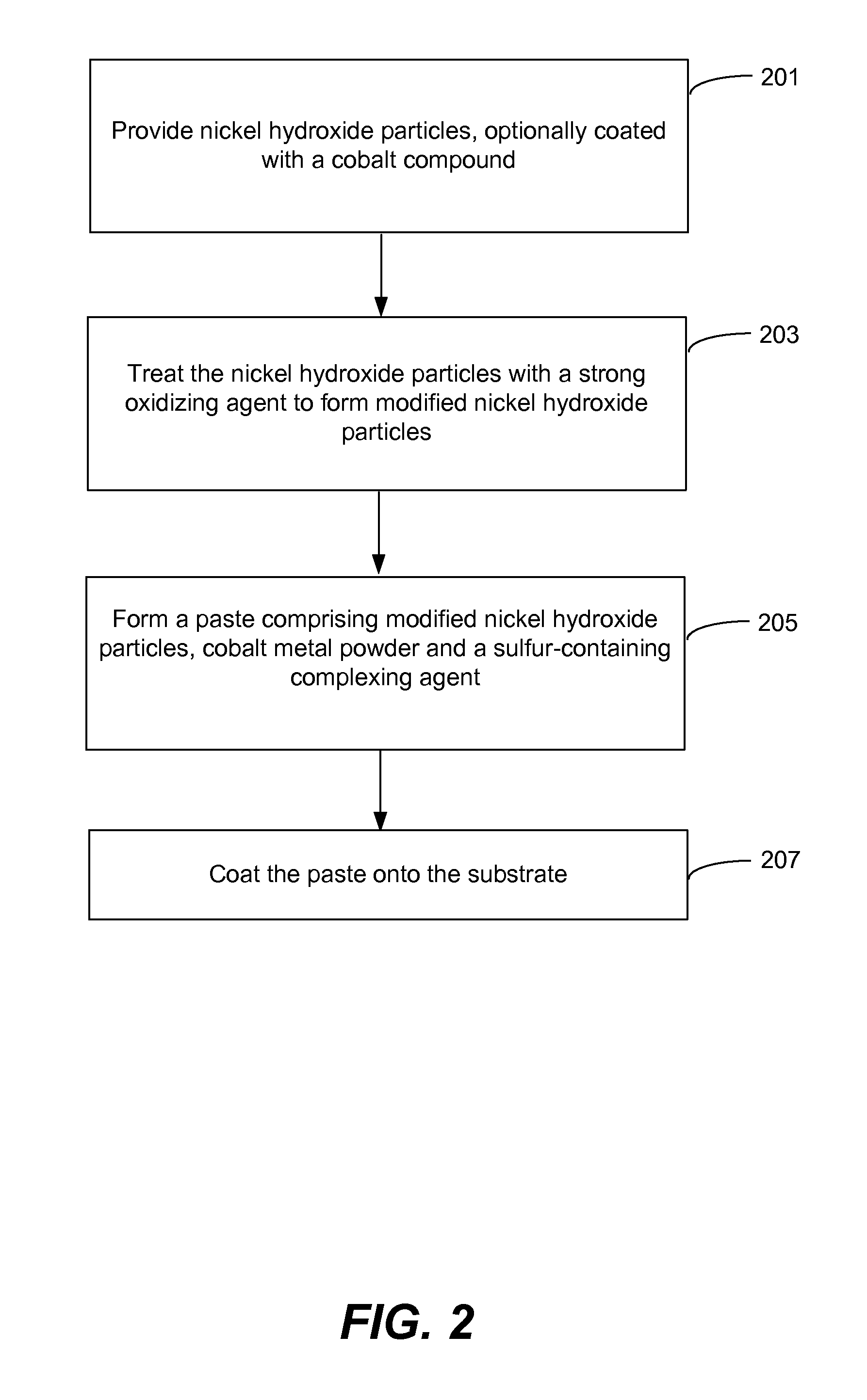
Nickel hydroxide electrode for rechargeable batteries
ActiveUS20090208839A1Facilitate interface reactionImprove reliabilityMaterial nanotechnologyHybrid capacitor electrodesPotassium persulfateNickel oxide hydroxide
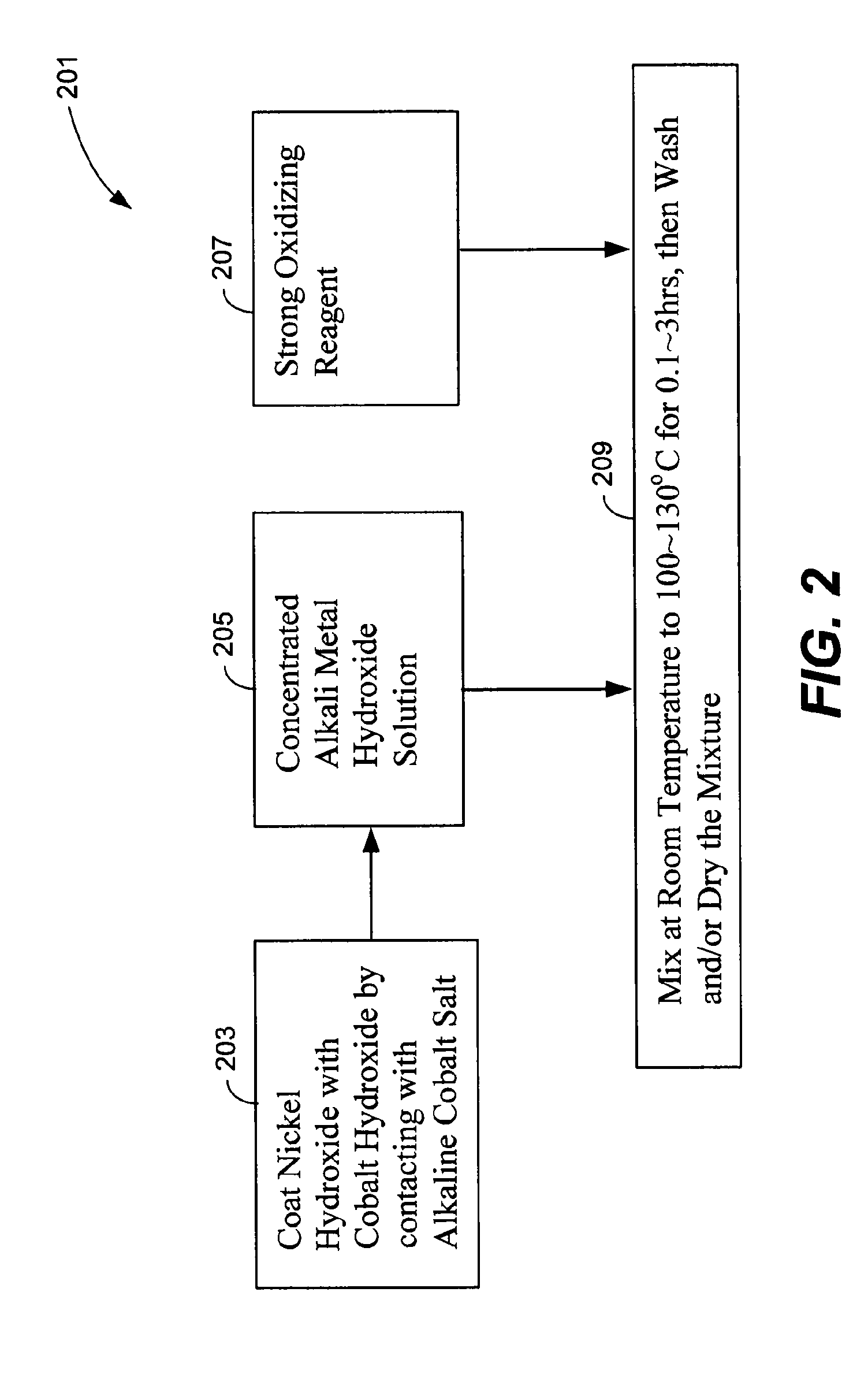
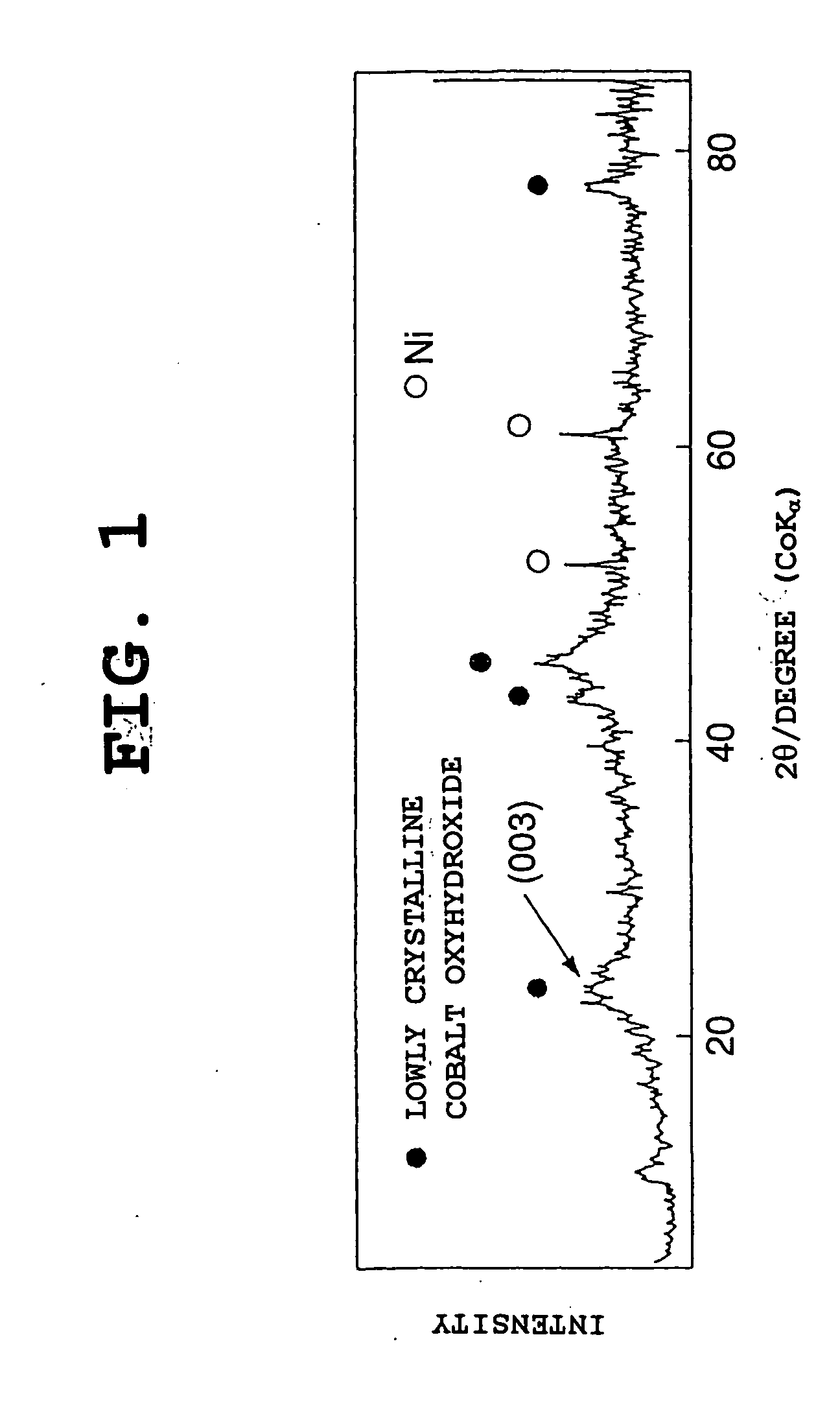
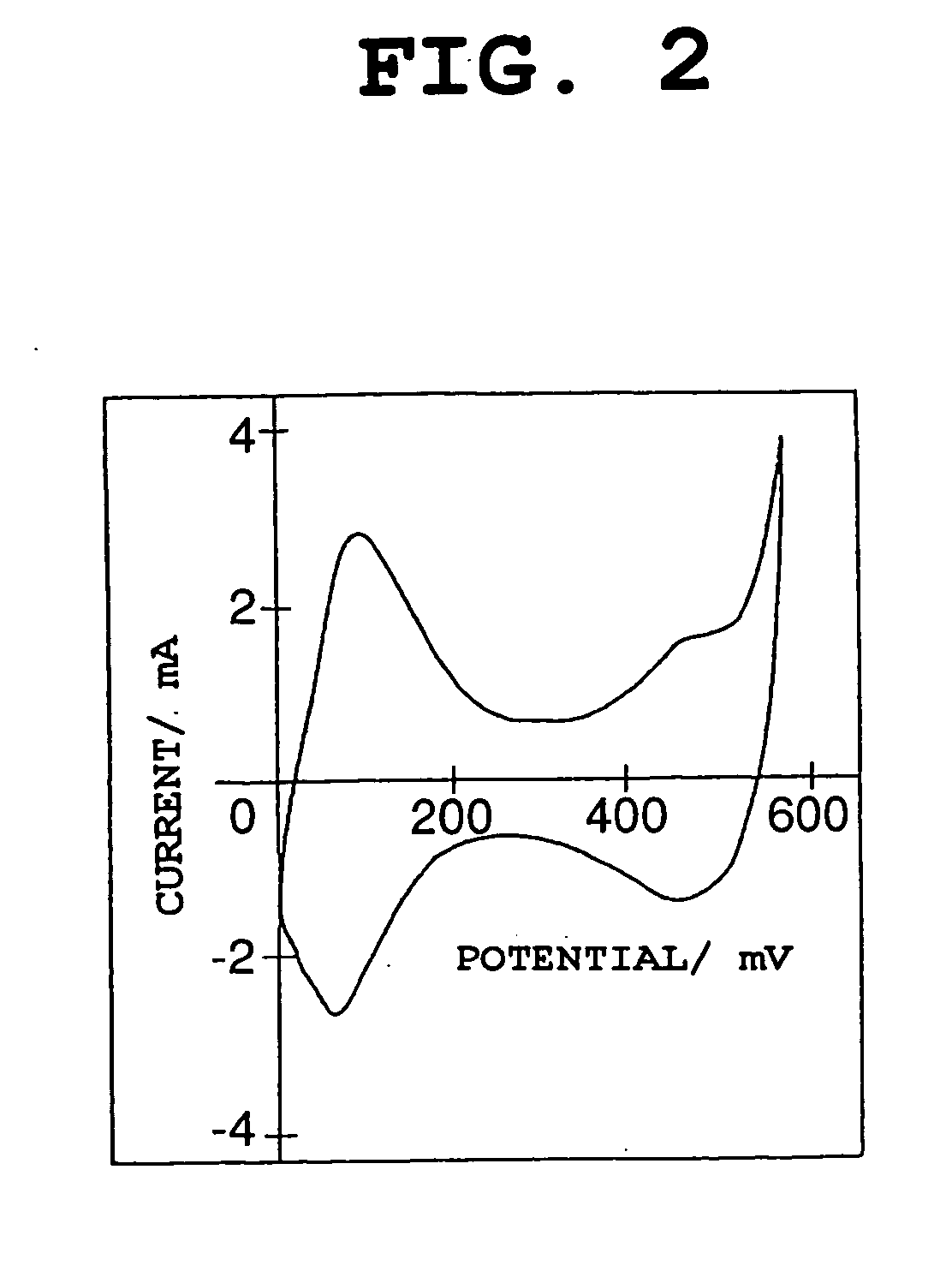
The nickel hydroxide particles for a nickel hydroxide electrode may be treated using an alkaline solution of a strong oxidizing agent such as sodium or potassium persulfate to modify the surface nickel hydroxide structure. The resulting modified surface structure has been found to impart various benefits to electrodes formed from the nickel hydroxide. It is believed that the oxidation of cobalt compounds at the surface of the nickel hydroxide particles results in a highly conductive cobalt compound that plays an important role in the high reliability, high stability and high capacity utilization of nickel electrodes as described herein.
Owner:ZINCFIVE POWER INC
Lithium-rich manganese-based anode material and method for manufacturing same
ActiveCN102916169AImprove featuresUniform structureCell electrodesSecondary cellsPtru catalystActive agent
The invention discloses a lithium-rich manganese-based anode material and a method for manufacturing the same. The method includes steps of (a), providing mixed solution containing lithium compounds, nickel compounds and manganese compounds, optional titanium compounds, optional iron compounds, optional cobalt compounds or an optional combination of the titanium compounds, the ion compounds and the cobalt compounds; (b), adding complexing agents, catalysts and surfactants into the mixed solution to form pre-coagulated substances; and (c), calcining the pre-coagulated substances to obtain the lithium-rich manganese-based anode material Li[LixNiaMnbM1-a-b-x]O2 or a combination of lithium-rich manganese-based anode materials. The complexing agents, the catalysts and the surfactants are used for forming the pre-coagulated substances, the complexing agents contain resorcinol and formaldehyde, in the molecular formula of the lithium-rich manganese-based anode material, the M represents Ti, Fe, Co or a combination of the Ti, the Fe and the Co, the x is larger than 0 and is smaller than or equal to 0.4, the a is larger than 0 and is smaller than or equal to 0.5, the b is larger than or equal to 0.33 and smaller than or equal to 0.6, and a result of 1-a-b-x is larger than or equal to 0. The lithium-rich manganese-based anode material is of a multi-channel porous structure, is small in grain size, uniform in grain distribution, advanced in porosity and stable in electrochemical performance.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Manganese nickel cobalt composite lithium-inserting oxide and manufacturing method thereof
InactiveCN1547277AImprove cycle stabilityIncrease capacityElectrode thermal treatmentActive material electrodesLithium oxideManganese
The invention is lithium ion cell positive material and the manganese-nickel-cobalt compound embedded lithium oxide and the manufacturing method, the chemical formula is: Li0.7-1.0MnxNiyCozO2, x+y+z=1, x=0.2-0.5, x / y=0.8-1.2, z / x=0.1-1, the crystal structure is hexagonal system, the manufacturing method is: the mol proportion is Mn:Ni:Co=1:0.8-1.2:0.1-1, the compound liquid of the Mn2+, Ni2+, and Co2+ is confected, then they are heated, adds in excess alkali, the compound hydroxide is deposited and separated; the compound oxide can be acquired through baking and decomposition; the lithium material and the manganese-nickel-cobalt compound oxide is pressed after blended according to proportion of Li:(Mn+Ni+Co)=0.7-1.0:1, they are baked in oxide atmosphere under temperature of 700-1000oC for 6-36 hours, then they are cooled and crushed into the product. The process is simple, the capacity of the lithium ion cell positive material is large, the circular performance is good, and the cost is low.
Owner:HUNAN JINGXIN TECH
Plastic lens and process for preparing the lens
ActiveUS20050068492A1Inhibit coloringImprove scratch resistanceOrganic chemistryOptical articlesUltraviolet lightsDiethylene glycol
A plastic lens which absorbs ultraviolet light having wavelength of about 400 nm and suppresses coloring and a process for producing the lens. A plastic lens may be made from a composition which comprises (A) a lens material monomer comprising diethylene glycol bisallylcarbonate, (B) an organic peroxide-based polymerization initiator, (C) a cobalt compound represented by at least one of CoO.Al2O3 and Co.Al2O4, and (D) at least one ultraviolet light absorbent selected from 2-hydroxy-4-octyloxy-benzophenone, 2,2′,4,4′-tetrahydroxy-4-octyloxybenzophenone and 2,2′,4′-trihydroxy-4-octyloxybenzophenone. A process for producing a plastic lens comprises mixing component (A), component (B), a cobalt fluid comprising component (C) in a dispersant and component (D) and a step of casting the mixed fluid into a mold and polymerizing the fluid to obtain a plastic lens.
Owner:HOYA CORP
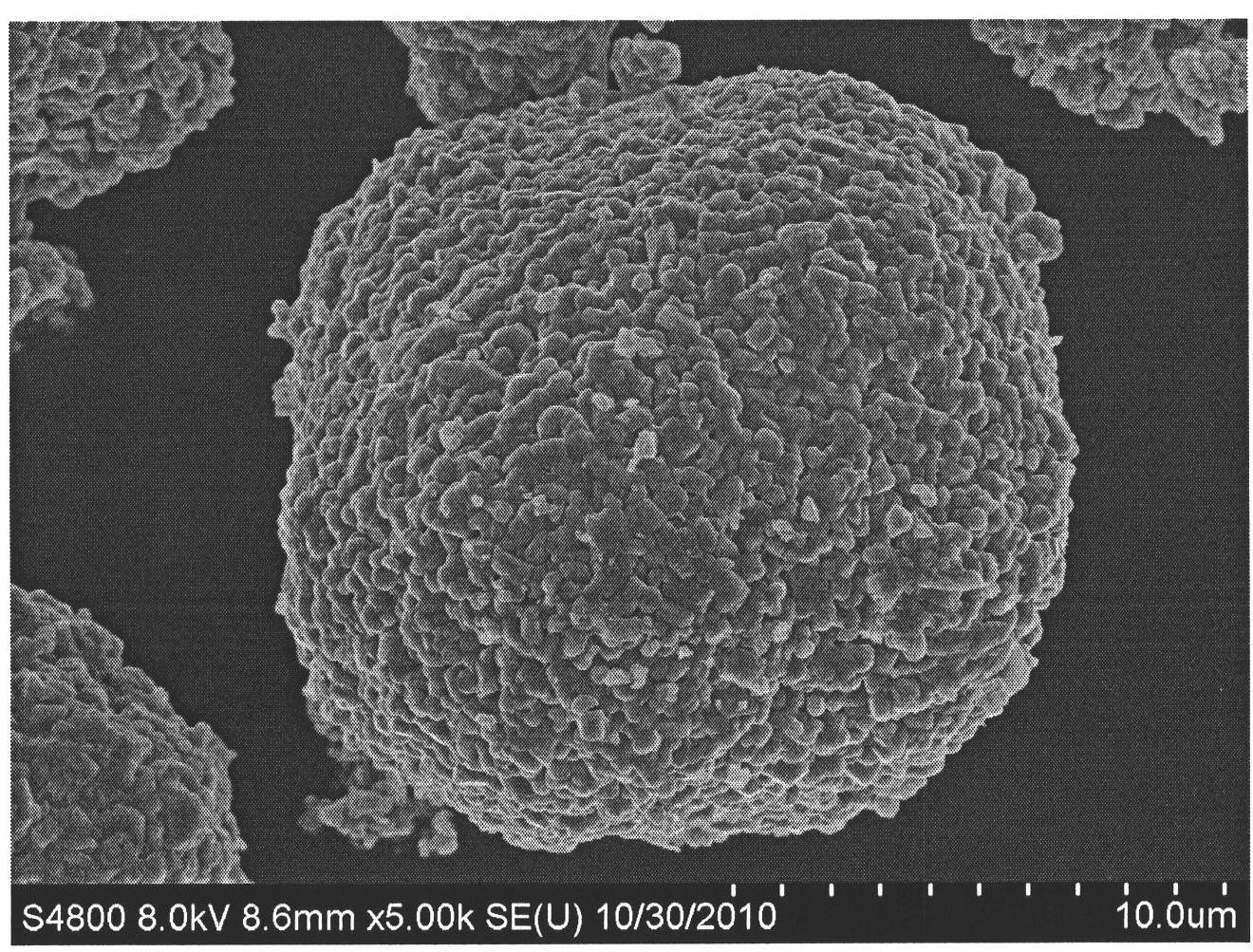
Dry preparation of battery grade spherical cobaltosic oxide particle
InactiveCN101434417AHigh densityUniform particle size distributionCell electrodesCobalt oxides/hydroxidesCobalt(II,III) oxideCobalt salt
The invention relates to a wet preparation method of battery-level spherical cobaltosic oxide, which comprises the specific steps of: preparing cobalt salt into a first mixed solution with the concentration of 1-3mol / L; preparing a hydrate solution with the concentration of 2-10mol / L into a second mixed solution; preparing a complexing agent A with the concentration of 0.001-200g / L into a third mixed solution; introducing the three mixed solutions into a reaction vessel by adopting a parallel flow method; stirring the mixed solution intensively to cause the three solutions to react; adding a certain amount of oxidizer continually into a reaction system during the reaction process; aging the mixed solution for 2-48 hours under normal temperature; centrifugalizing, washing and drying the material; and calcining the mixture of a pretreated precursor and lithium salt under 750-1000 DEG C to obtain the lithium battery cathode material. The method has the advantage that the cobaltosic oxide powder can be directly obtained from the oxidation of a cobalt compound in the solution owing to the fact that the cobalt compound can be oxidized in the solution under alkaline condition. The material is a relatively ideal battery cathode material for mobile telephones, video cameras, laptops and portable electric appliances.
Owner:NINGBO JINHE NEW MATERIALS
Preparation method of nano-structure WC-Co composite powder
InactiveCN102350506AWell mixedOvercoming coarse compound salt crystallizationChromium CompoundsVanadium oxide
The invention discloses a preparation method of a nano-structure WC-Co composite powder, and belongs to the field of an alloy preparation method. The method comprises the following steps in sequence: adding a carbonic powder material which is excessive to tungsten, vanadium and chromium carbides into water-soluble saturated mixed aqueous solution of tungsten-containing compound, cobalt-containing compound, chromium-containing compound and vanadium-containing compound to adsorb the saturated composite salt solution; dehydrating and drying to form a nano-scale composite salt thin layer on the surface of the carbonic powder material; removing crystal water out of the composite salt at the temperature below 500 DEG C under a condition of isolating air, and decomposing the composite salt into composite oxide of tungsten oxide, chromium hemitrioxide, vanadium oxide and cobalt oxide, further heating, and reducing and carbonizing the composite oxide on the surface of the carbonic powder material to generate the WC-Co nano-structure composite powder at the temperature below 850 DEG C. The preparation is a simpler and more reliable novel preparation method of the nano WC-Co composite powder by a direct carbonization method.
Owner:SOUTHWEST PETROLEUM UNIV
Process for Production of Carboxylic Acid Ester or Ether Compound
InactiveUS20090012324A1Easy to operateEconomical to useOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholNickel compounds
Disclosed is a process for production of a carboxylic acid ester from a carboxylic acid and an olefin or production of an ether compound from an alcohol and an olefin at low cost and with high yield in an industrially advantageous manner. The process comprises the step of reacting a carboxylic acid with an olefin to yield a corresponding carboxylic acid ester or reacting an alcohol with an olefin to yield a corresponding ether compound. In the process, a catalyst comprising a combination of (i) at least one metal compound selected from an iron compound, a cobalt compound and a nickel compound and (ii) an acidic compound is used.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Current Collectors for Solid Oxide Fuel Cell Stacks
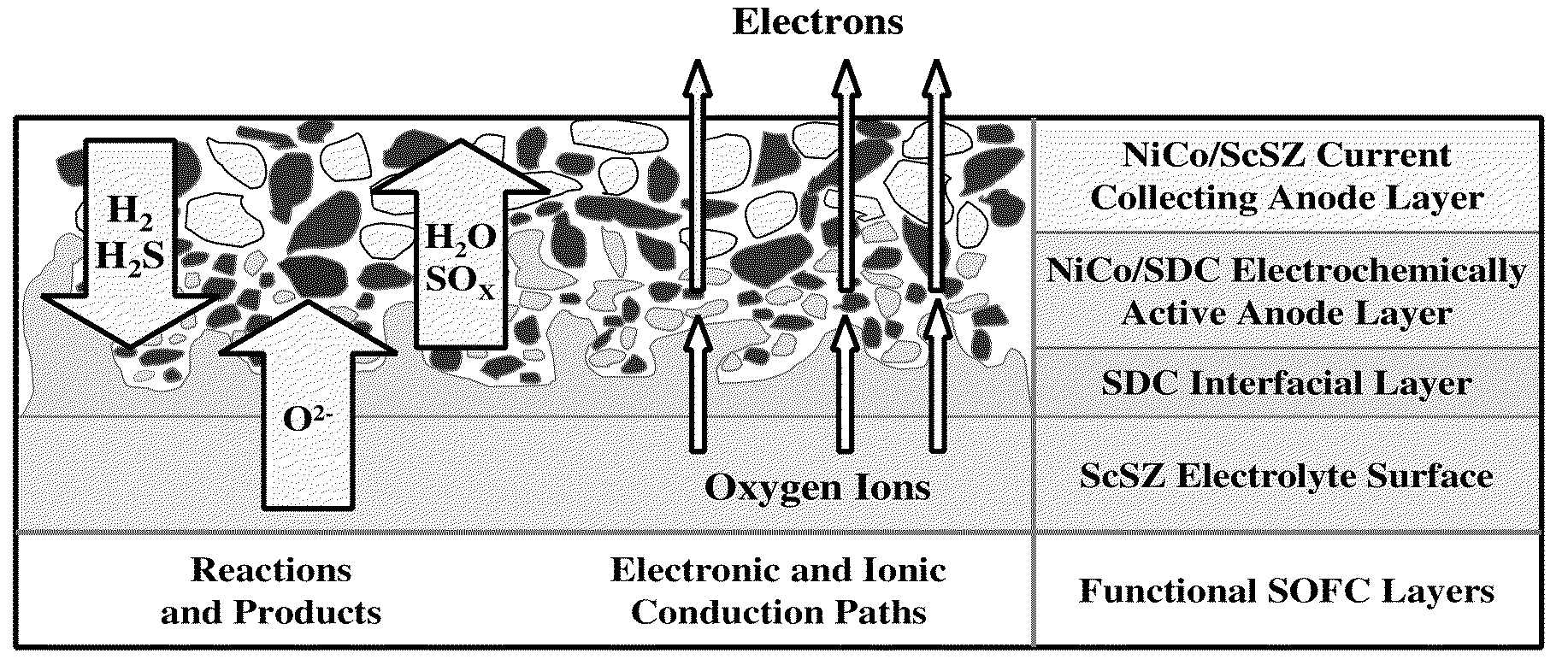
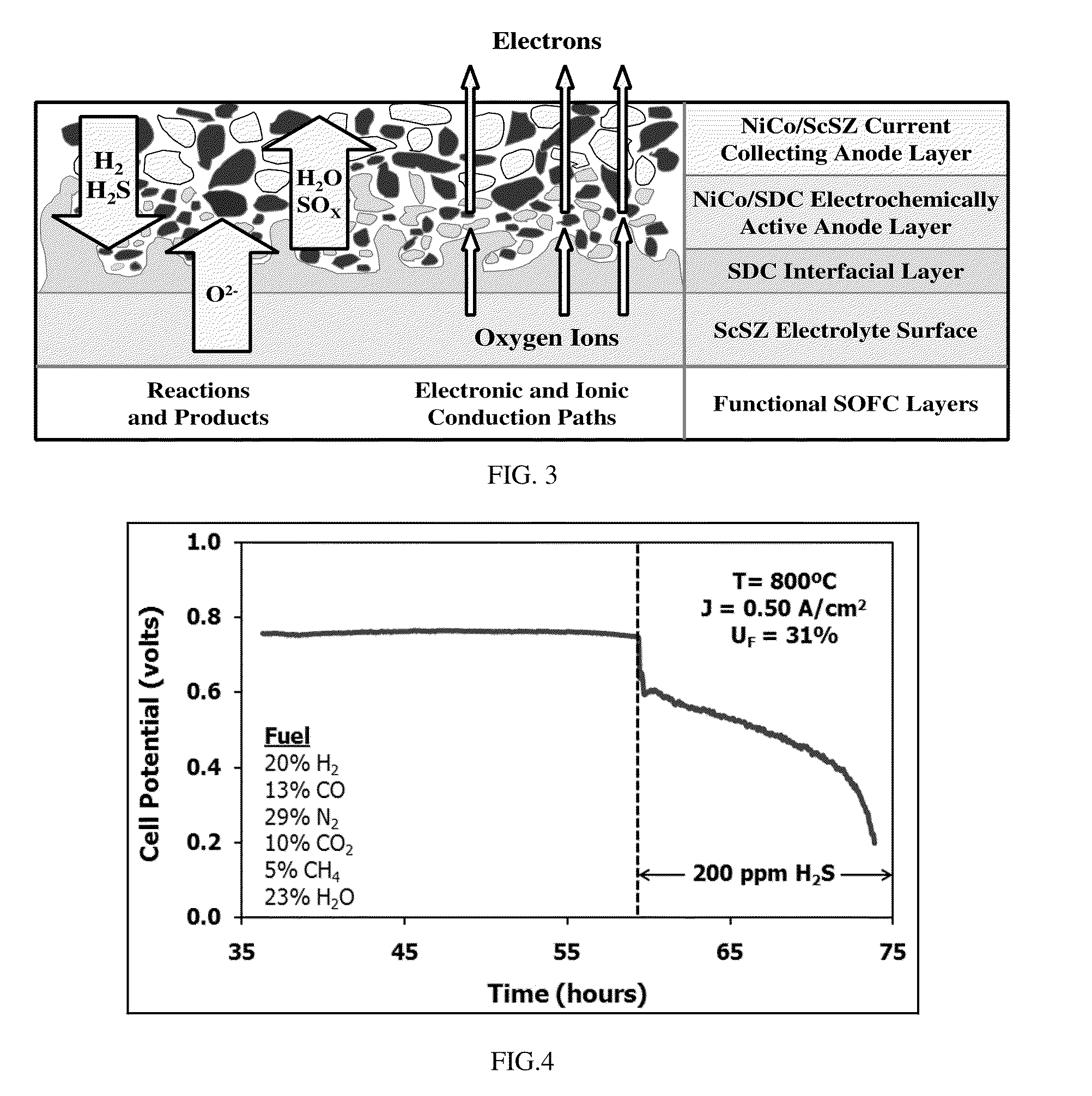
A sulfur tolerant anode current collector material includes a mesh or foam that includes a cermet. The cermet includes a metallic component and a ceramic component. The metallic component includes nickel, an alloy including nickel and cobalt, or a mixture including a nickel compound and a cobalt compound. The ceramic component includes a mixed conducting electrolyte material.
Owner:NEXCERIS INNOVATION HLDG LLC
Layered perovskite structural ceramic and preparation method thereof
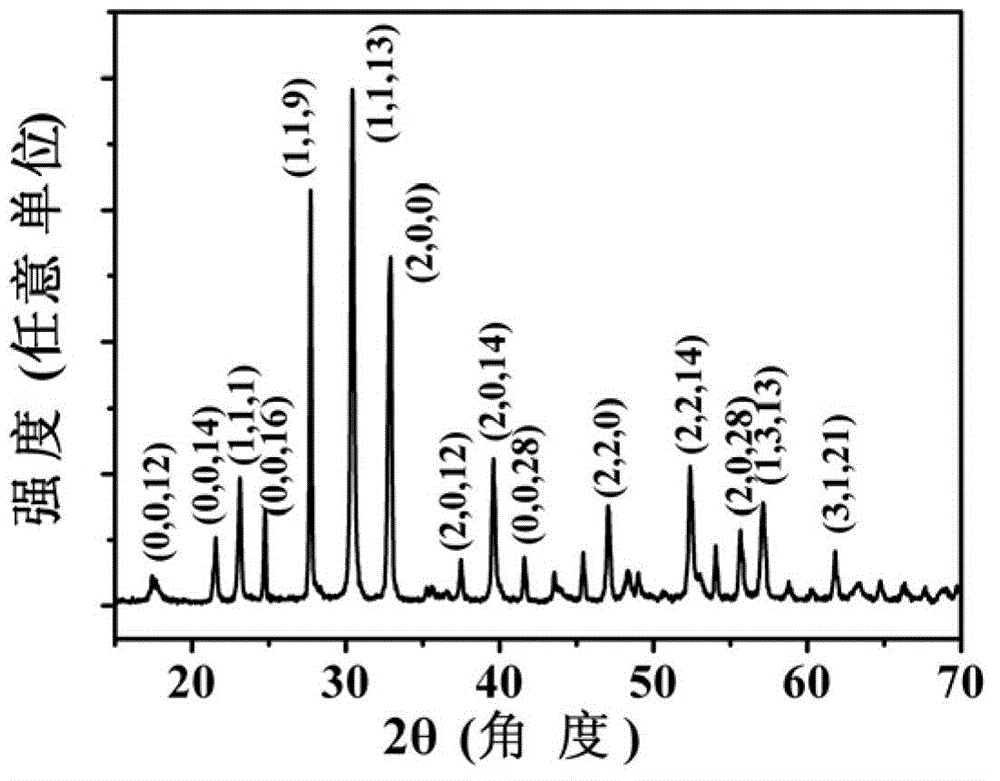
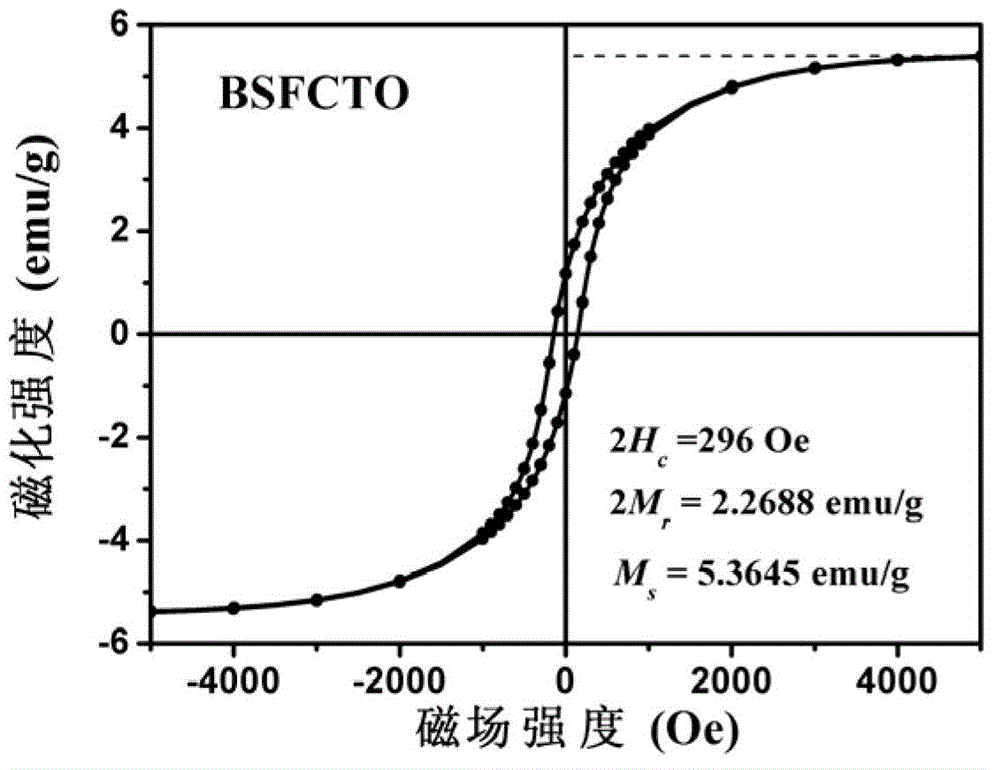
The invention provides layered perovskite structural ceramic and a preparation method thereof. The preparation method includes mixing well titanate compounds, bismuth compounds, strontium compounds, iron compounds, cobalt compounds and complexing agent in solvent, heating, drying and combusting to obtain powder, and presintering to obtain powder; performing the powder, and performing hot-pressing sintering to obtain the structural ceramic in in a formula (I). Compared with ceramics prepared in the prior art by solid phase sintering process, the structural ceramic in the formula (I) is prepared by solution process and hot pressing sintering. The preparation method has the advantages that less volatile low-valence strontium ions are introduced, increased leakage current due to volatilization of the strontium ions is improved, and ferroelectric properties are improved; the raw materials are evenly spread by the solution process, the power is even in component and has high reactivity, and Fe-Co ions are coupled fully; and the materials processed by hot pressing sintering are high in density and orientation, and the layered perovskite structural material can be obtained, namely Bi(n+1-x)SrxF3(n-3) / 2Co(n-3) / 2Ti3O3(n+1) (I).
Owner:UNIV OF SCI & TECH OF CHINA
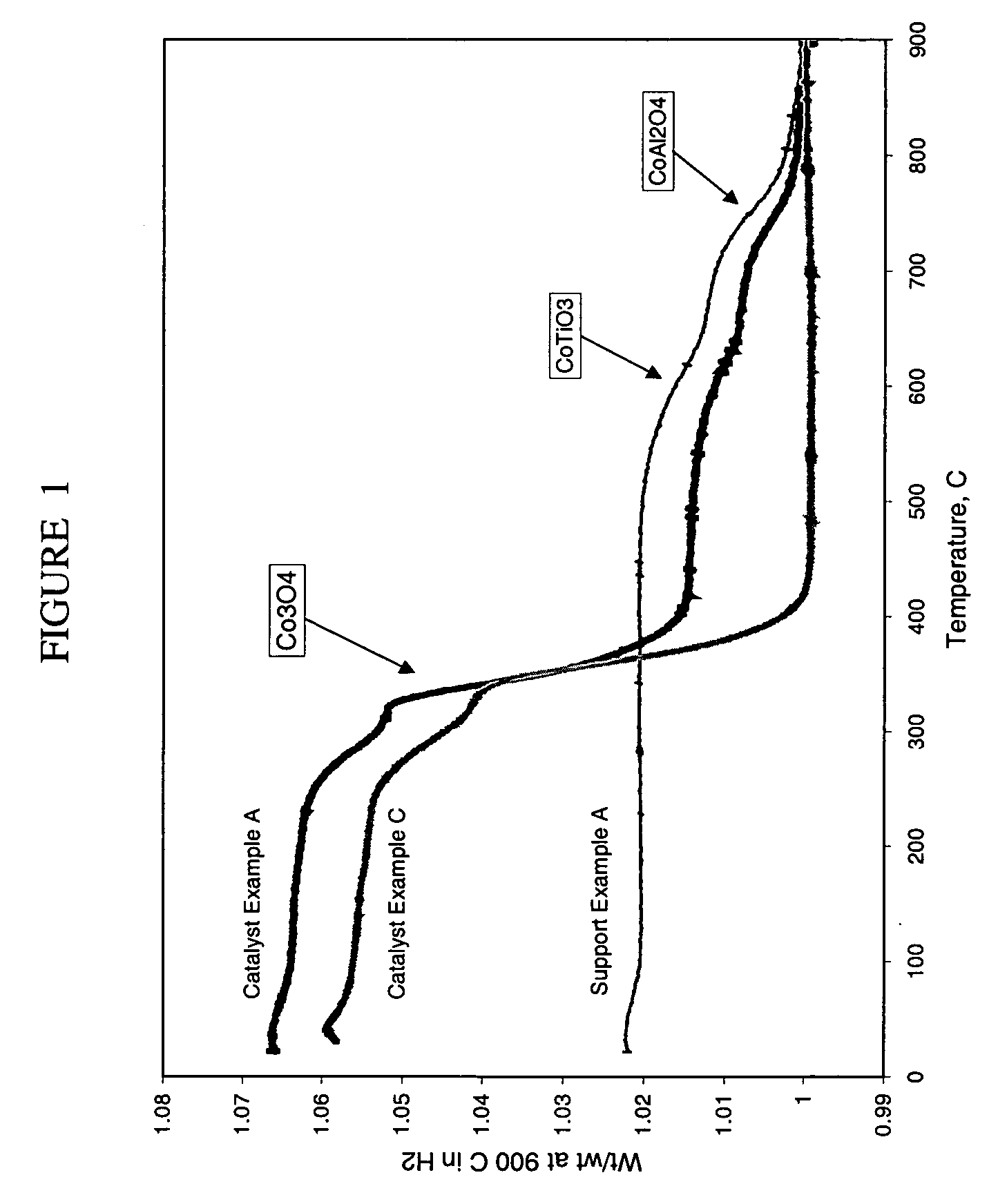
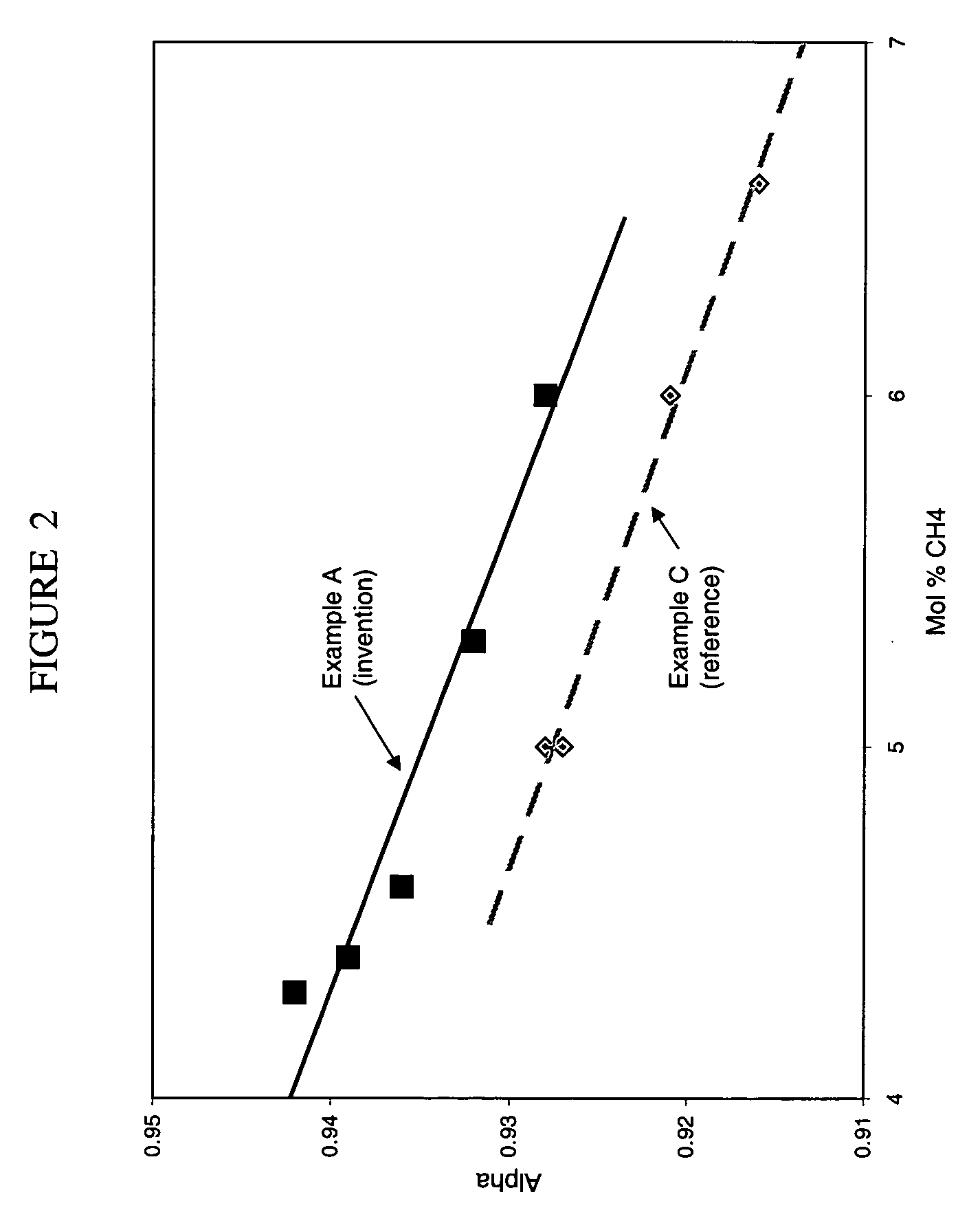
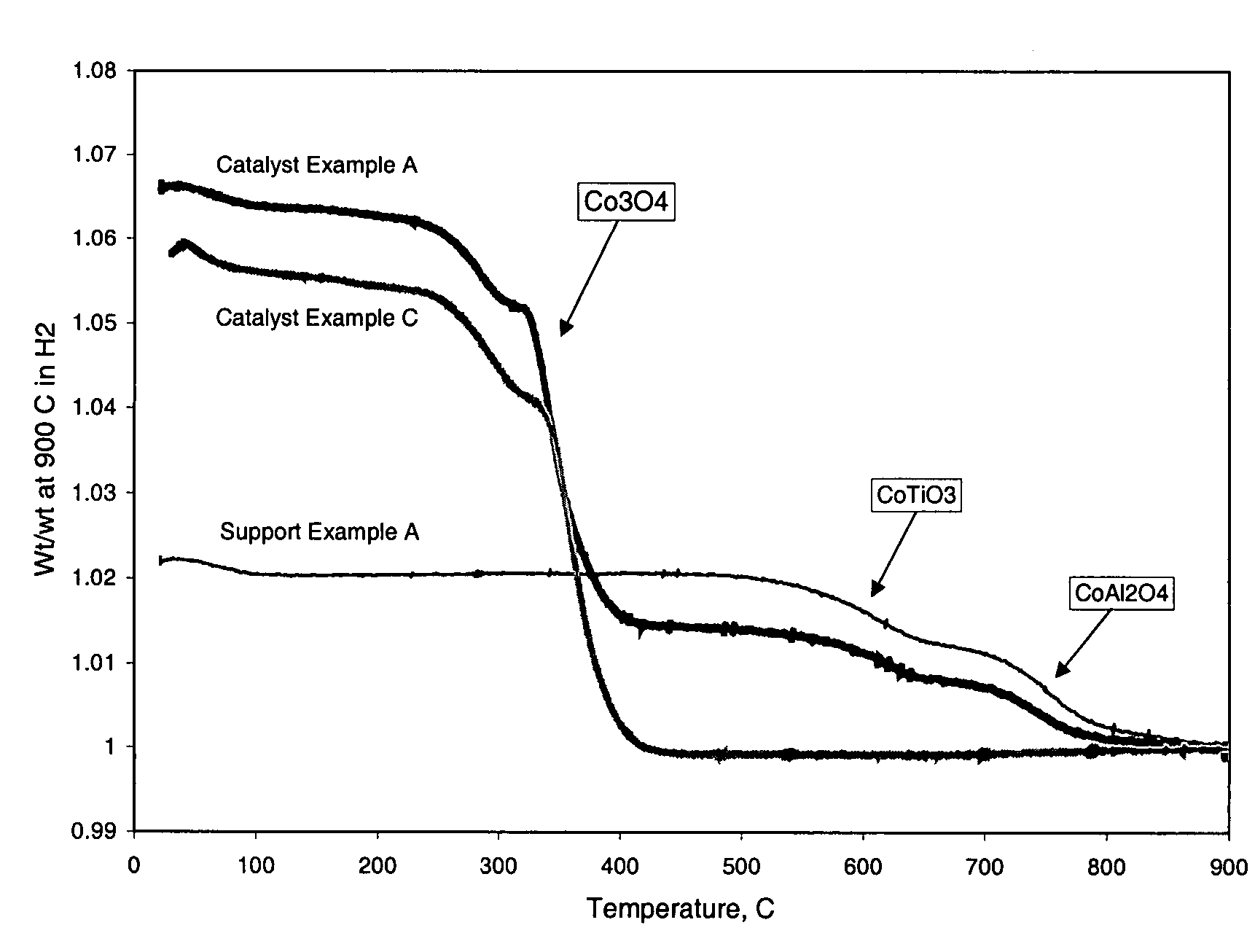
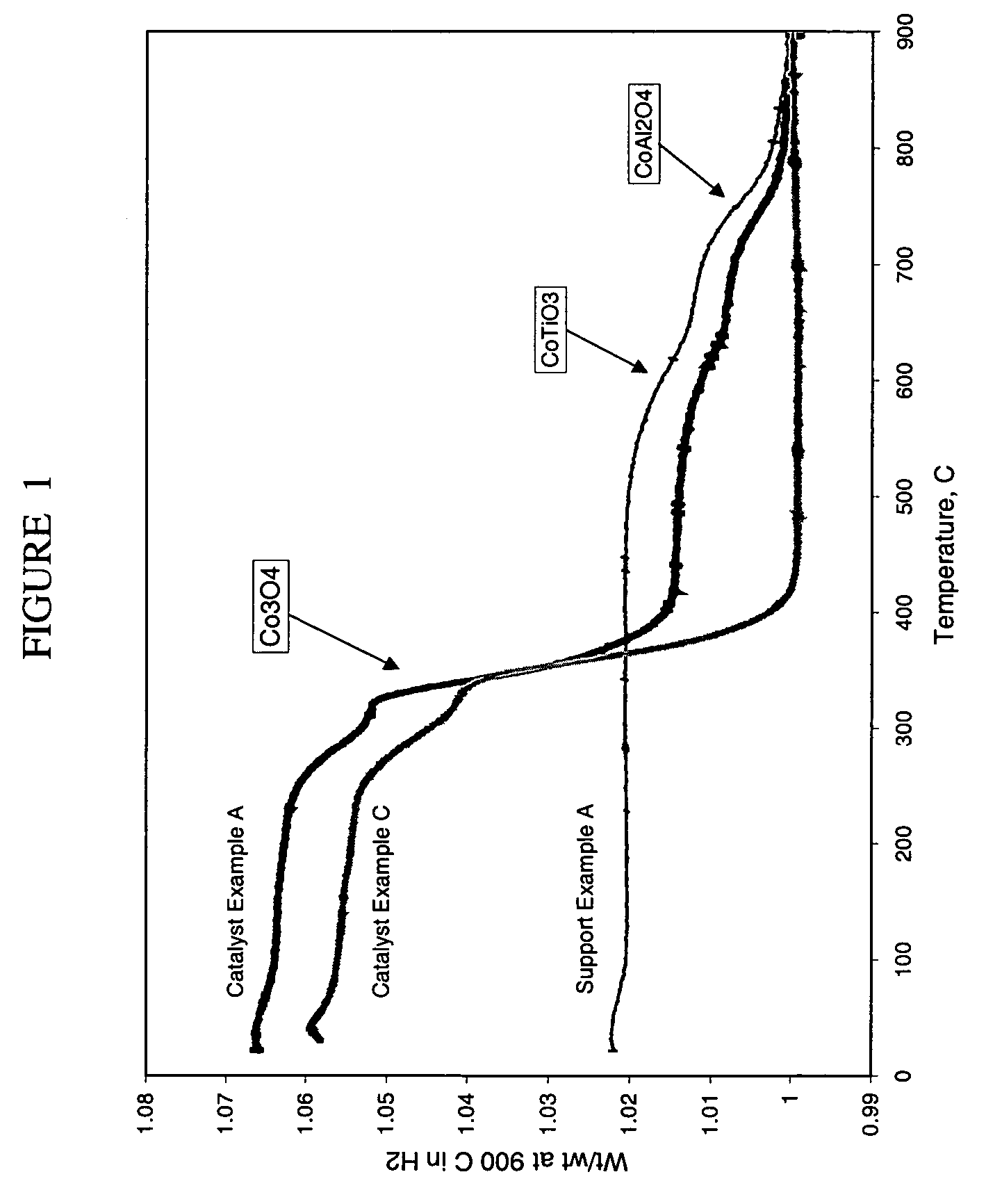
Preparation of titania and cobalt aluminate catalyst supports and their use in Fischer-Tropsch synthesis
ActiveUS20040204506A1Add supportOther chemical processesOrganic compound preparationSilicon dioxideCobalt aluminate
Supports for Fischer-Tropsch catalysts are formed by forming a particulate material from titania, alumina and optionally silica. A cobalt compound is incorporated into the particulate material which then is calcined to convert at least part of the alumina to cobalt aluminate.
Owner:EXXON RES & ENG CO
Method for preparing lithium-rich solid solution cathode material through reduction
ActiveCN102881874ALow costWide variety of sourcesCell electrodesHybrid/EDL manufactureNickel compoundsManganese
The invention relates to a method for preparing a lithium-rich solid solution cathode material through reduction. The method is characterized by comprising the following steps of: weighing a lithium compound, a nickel compound, a manganese compound, a cobalt compound and a reducing agent in a molar ratio of lithium ions to nickel ions to manganese ions to cobalt ions to the reducing agent of (1+x):(1-x).y:(x+z-x.z):(1-x).k:q; and mixing the reducing agent and a wet grinding medium, mixing the weighed nickel compound, manganese compound and cobalt compound in a mixture, performing wet grinding and mixing, adding ammonia water, performing wet grinding again, aging, drying, putting a precursor in air, oxygen-rich gas or pure oxygen atmosphere, and performing two-section or two-time sectional sintering to prepare the lithium-rich solid solution cathode material xLi2MnO3.(1-x)Li[NiyMnzCok]O2. The electrode material prepared by the method has uniform composition, high discharge performance, and high discharge cycle performance particularly under the high-current condition.
Owner:FUJIAN NORMAL UNIV
Mixed ligand cobalt (ii) complex and its preparation method and application
InactiveCN102276658AImprove thermal stabilityHigh yieldCobalt organic compoundsMagnitude/direction of magnetic fieldsMagnetic transitionsPhysical chemistry
The invention relates to a 1,2,4-triazole and 5-sulfonyl m-phthalic acid mixed ligand cobalt (II) coordination compound magnetic material as well as a preparation method and application thereof. The chemical formula of the coordination compound magnetic material is {[Co3.5(H2O)2(trz)4(sip)].1.5H2O}, wherein trz is 1,2,4-triazole monovalent anion; and sip is trivalent anion of 5-sulfonyl m-phthalic acid. The coordination compound magnetic material is prepared by using a solvothermal method and has higher yield and good repeatability. The coordination compound magnetic material is the first case of cobalt (II) compound containing the 1,2,4-triazole and 5-sulfonyl m-phthalic acid mixed ligand, shows a magnetic transition phenomenon induced by a three-step field under different external magnetic fields, can be used as a molecule-based magnetic material and has great application value in the field of material science.
Owner:TIANJIN NORMAL UNIVERSITY
Preparation of titania and cobalt aluminate catalyst supports and their use in Fischer-Tropsch synthesis
ActiveUS7253136B2Organic compound preparationOther chemical processesSilicon dioxideCobalt aluminate
Supports for Fischer-Tropsch catalysts are formed by forming a particulate material from titania, alumina and optionally silica. A cobalt compound is incorporated into the particulate material which then is calcined to convert at least part of the alumina to cobalt aluminate.
Owner:EXXON RES & ENG CO
Composite free layer for stabilizing magnetoresistive head
InactiveUS20070002503A1Reduce adverse effectsStable against spin transfer induced switchingNanomagnetismMagnetic measurementsDamping factorMagnetic reluctance
A magnetoresistive read head includes a spin valve having at least one free layer spaced apart from at least one pinned layer by a spacer. The free layer includes a cobalt compound as a thin film including at least one of Co—X, CoFe—X and CoNi—X, where X is an element from the lanthanoid family (a 4-f element). The content of Co is higher than 80 percent, and the content of the lanthanoid element is less than 10 percent. The film may comprise the entire free layer, or be positioned adjacent to one or more conventional free layer films. The pinned layer is a conventional single layer, or a synthetic multi-layered structure having a spacer between sub-layers. Because the spin valve structure has a high exchange stiffness and damping factor, spin transfer effect is reduced and a high-speed dynamic response is provided.
Owner:TDK CORPARATION
Method for preparing lithium-enriched solid solution cathode material by doping iron, copper and tin ions
The invention provides a method for preparing a lithium-enriched solid solution cathode material by doping iron, copper and tin ions, which is characterized in that a preparation process is formed by the following steps of: mixing a nickel compound, a manganese compound, a cobalt compound and an M compound, which are weighed to form a mixture 1; adding a wet milling medium and organic weak acid; carrying out wet milling and mixing and adding a lithium compound; after carrying out the wet milling and the mixing, utilizing a vacuum drying or spraying drying method to treat a precursor 1 to prepare a dry precursor 2; putting the precursor 2 into air, oxygen-enriched gas or pure oxygen atmosphere; and utilizing a two-section sintering method or a two-time subsection sintering method to prepare the lithium-enriched solid solution cathode material of xLi2MnO3*(1-x)Li[NiyMnzCokMq]O2. An electrode material prepared by the method disclosed by the invention is uniformly formed and has a very good discharging performance; and particularly, the electrode material has the excellent circulating discharging performance under a large-current condition.
Owner:DYNABAT NEW ENERGY SCI & TECH CO CLD FUJIAN
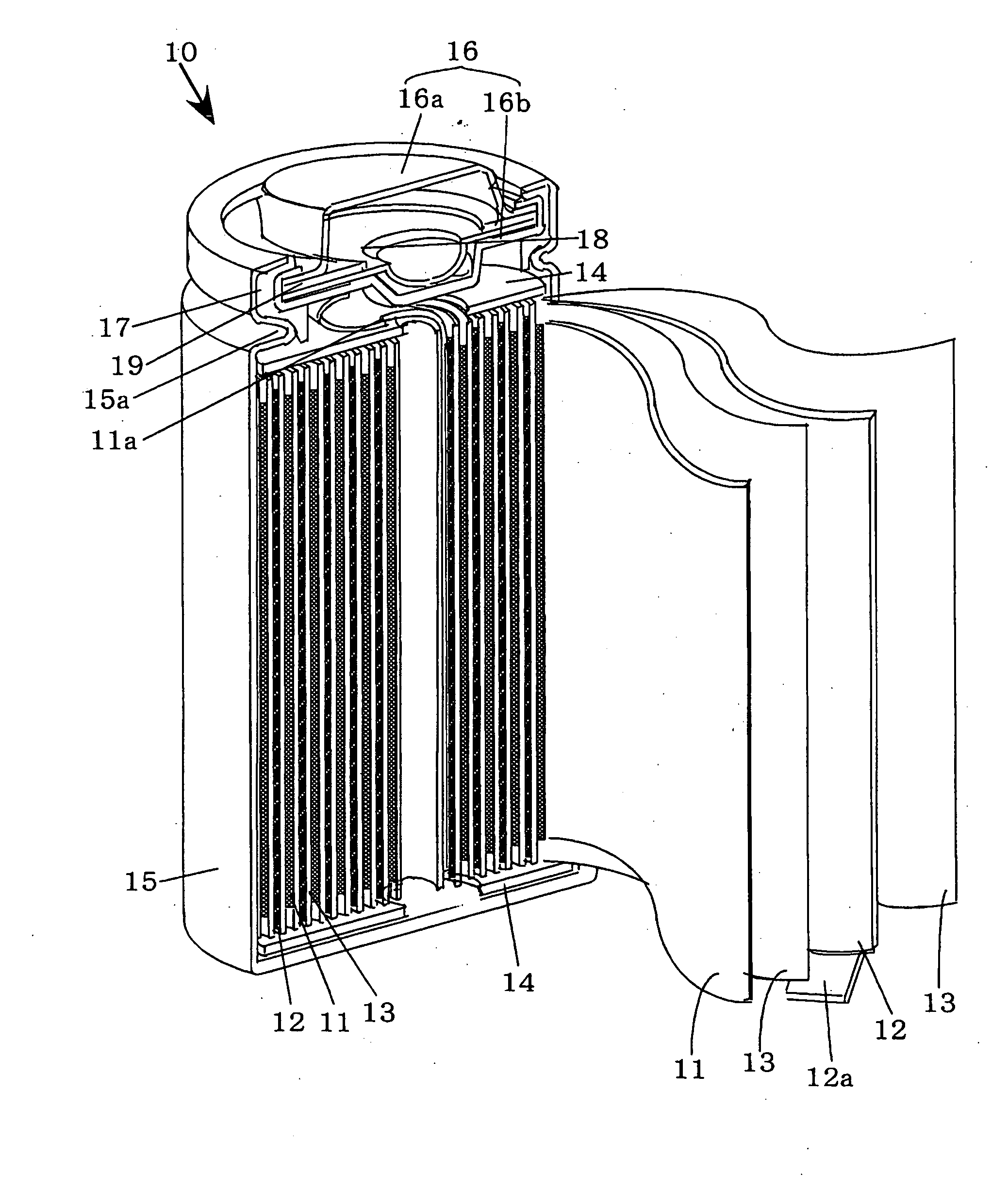
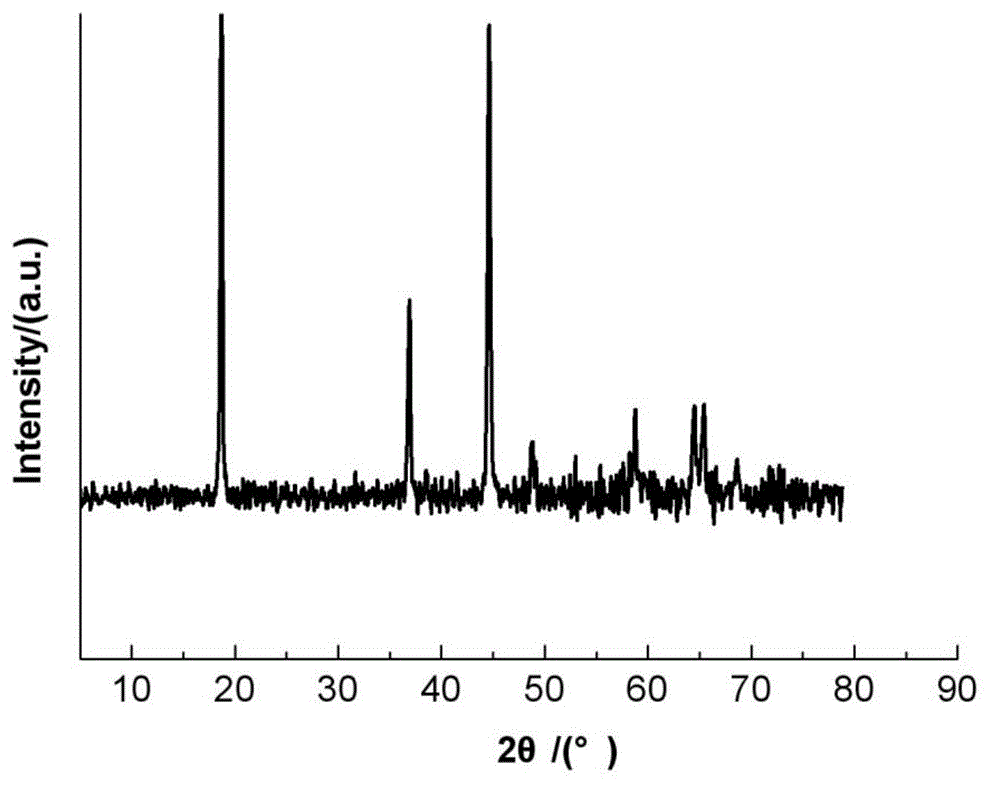
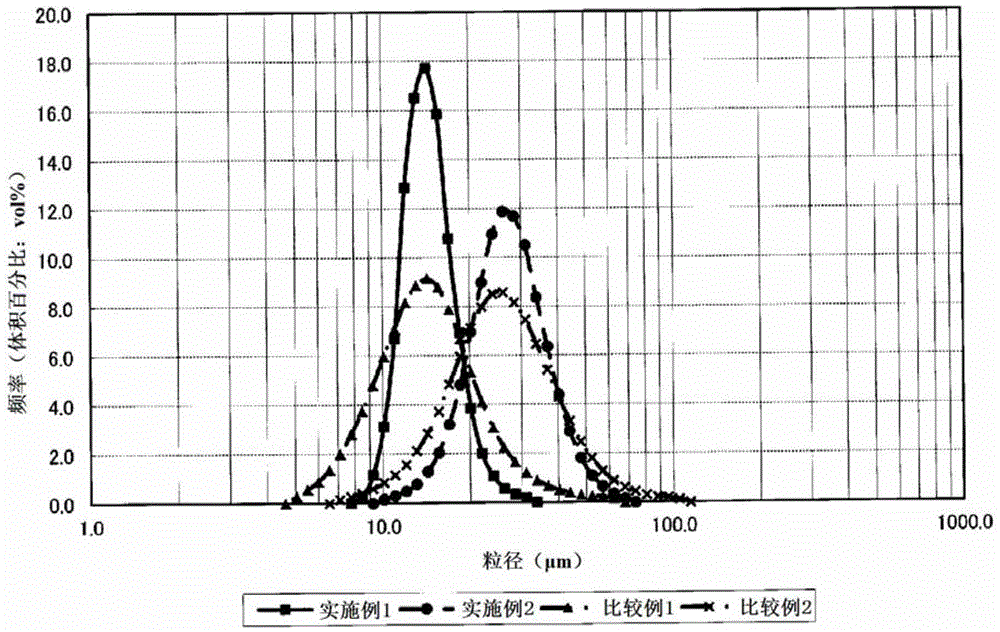
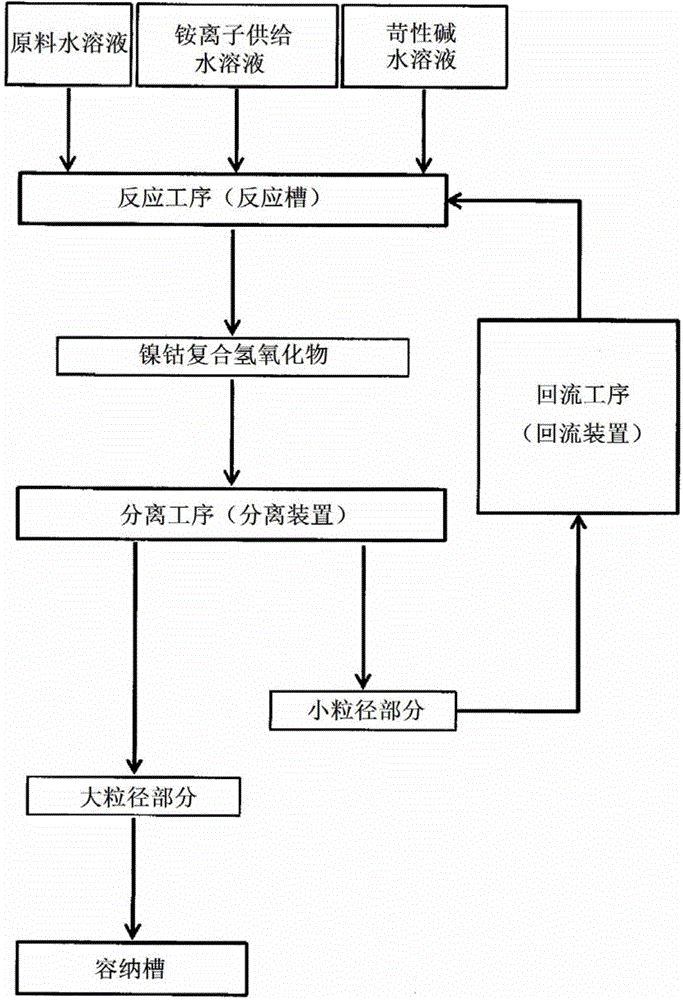
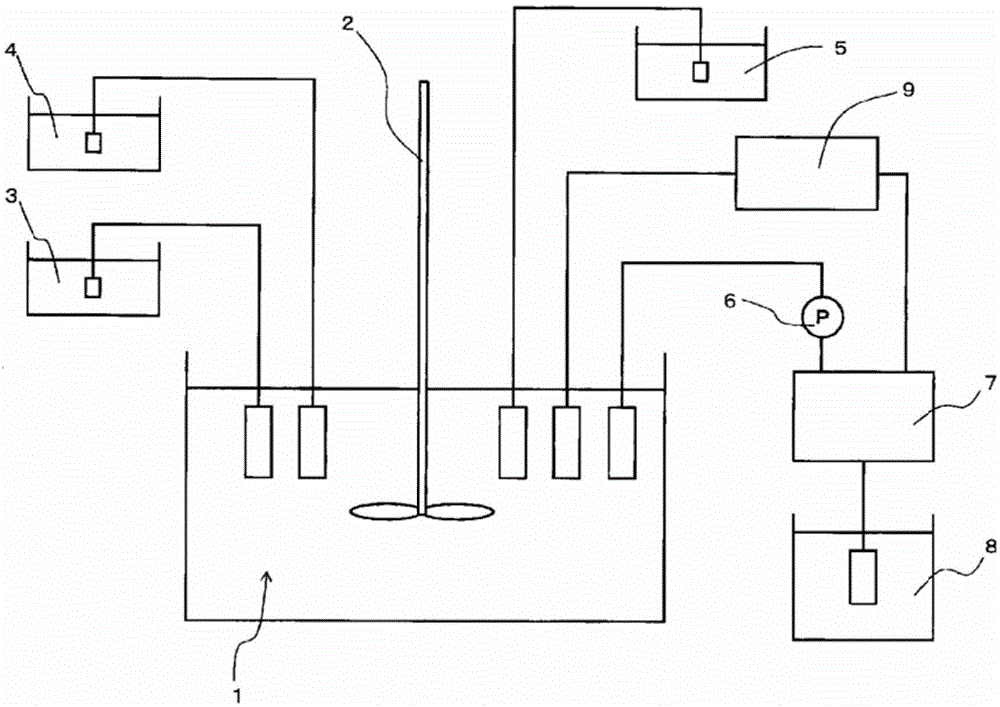
Nickel-cobalt compound hydroxide and method and device for producing same, positive electrode active substance for nonaqueous electrolyte secondary cell and method for producing same, and nonaqueous electrolyte secondary cell
ActiveCN104661963ASharp particle size distributionExcellent cycle characteristicsSecondary cellsChemical electrode manufacturingSlurryAqueous solution
[Problem] By obtaining a compound hydroxide that has a sharp particle diameter distribution, the present invention aims to improve the cycle properties of a nonaqueous electrolyte secondary cell obtained using the nickel-cobalt compound hydroxide as the precursor. [Solution] In the present invention, a slurry contains a nickel-cobalt compound hydroxide that is obtained by reacting and continuously feeding to a reactor an aqueous solution containing at least nickel and cobalt, an aqueous solution that serves as an ammonium ion donor, and an aqueous caustic alkali solution. The slurry is continuously extracted and the slurry is separated by grading into large particle diameter portions and small particle diameter portions. The small particle diameter portion is continuously circulated back to the reactor. The resulting nickel-cobalt compound hydroxide is represented by the general formula Ni1-x-yCoxMy (OH)2 (where 0.05 ≤ x ≤ 0.50, 0 ≤ y ≤ 0.10, 0.05 ≤ x + y ≤ 0.50, M is at least one metal element selected from Al, Mg, Mn, Ti, Fe, Cu, Zn, and Ga), wherein the correlations of (D50-D10) / D50 ≤ 0.30 and (D90-D50) / D50 ≤ 0.30 are established between D10, D50, and D90.
Owner:SUMITOMO METAL MINING CO LTD
Nickel electrode material,and production method therefor, and nickel electrode and alkaline battery
ActiveUS20040241545A1Improved cycle life characteristicsHigh degreeElectrochemical processing of electrodesLayered productsInternal pressureNickel oxide hydroxide
Subjects for the invention are to provide a nickel electrode material having a satisfactory tap density and capable of attaining a sufficient reduction in discharge reserve and a process for producing the nickel electrode material, and to provide a nickel electrode and an alkaline storage battery having a high capacity and excellent internal-pressure characteristics. For accomplishing the subjects, the invention provides a nickel electrode material for use in a nickel electrode, wherein the positive-electrode material constituting the electrode material comprises: positive active material particles which comprise as the main component either a nickel hydroxide or a solid solution in a nickel hydroxide of one or more other elements and in which part of the nickel hydroxide has been oxidized; and a coating layer formed on the surface of the positive active material particles and comprising as the main component a high-order cobalt compound in which the cobalt has an oxidation number larger than 2, the average oxidation number of the nickel in the positive active material particles and the cobalt in the coating layer being from 2.04 to 2.40, and the positive-electrode material having a tap density of 2.0 g / cm<3 >or higher. The invention further provides a process for producing the electrode material, a nickel electrode employing the electrode material, and an alkaline storage battery having the nickel electrode.
Owner:GS YUASA INT LTD
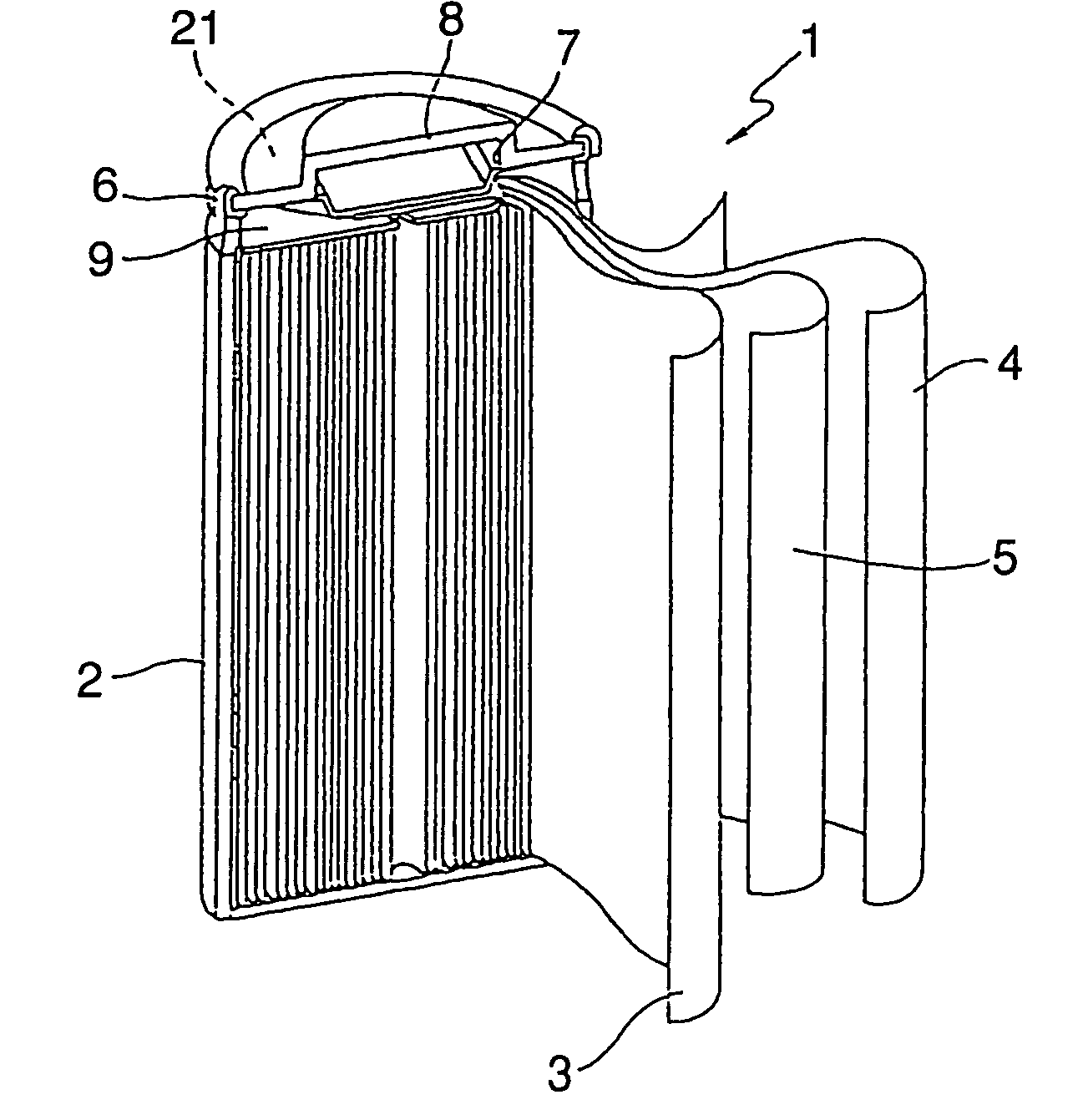
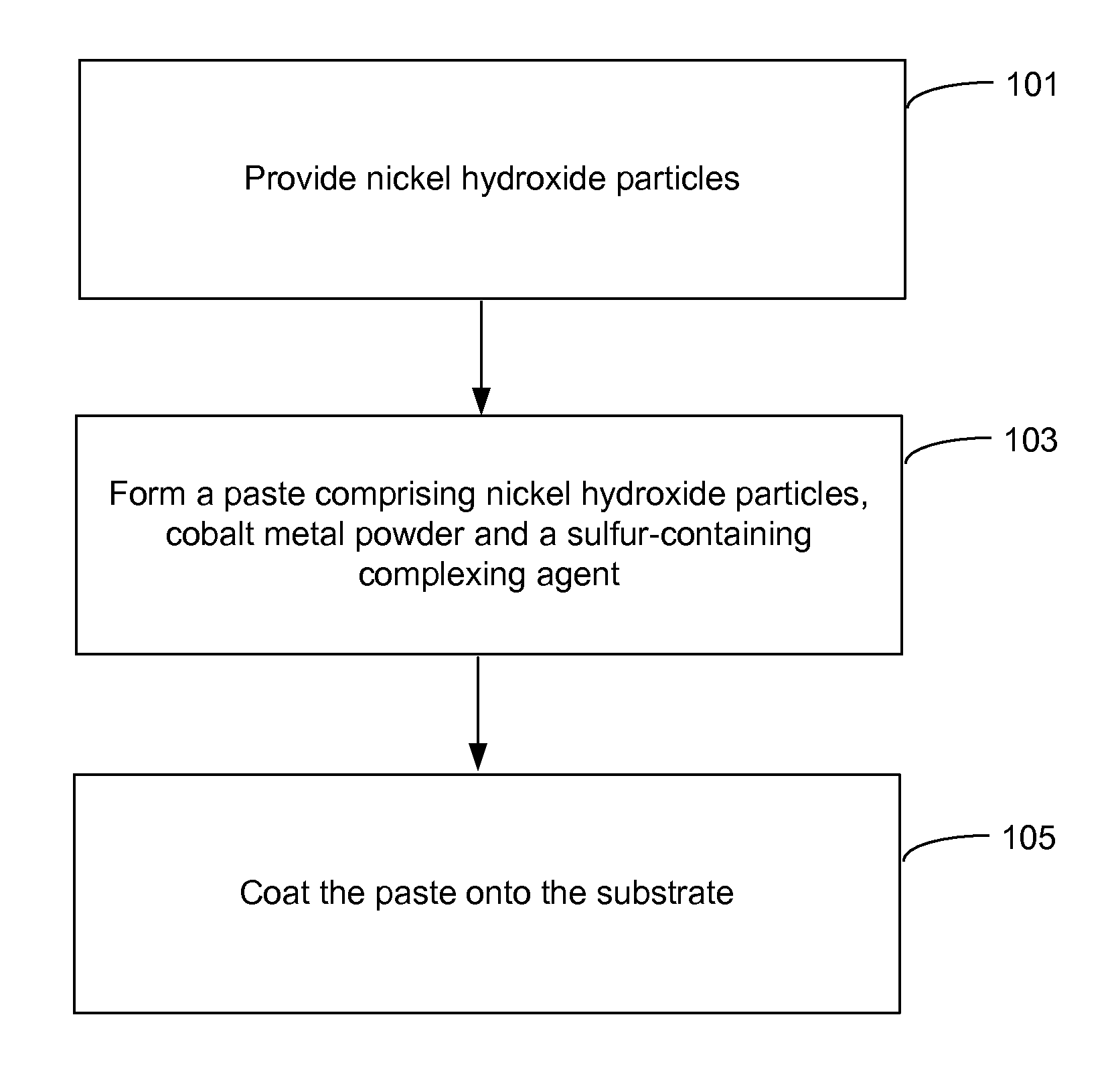
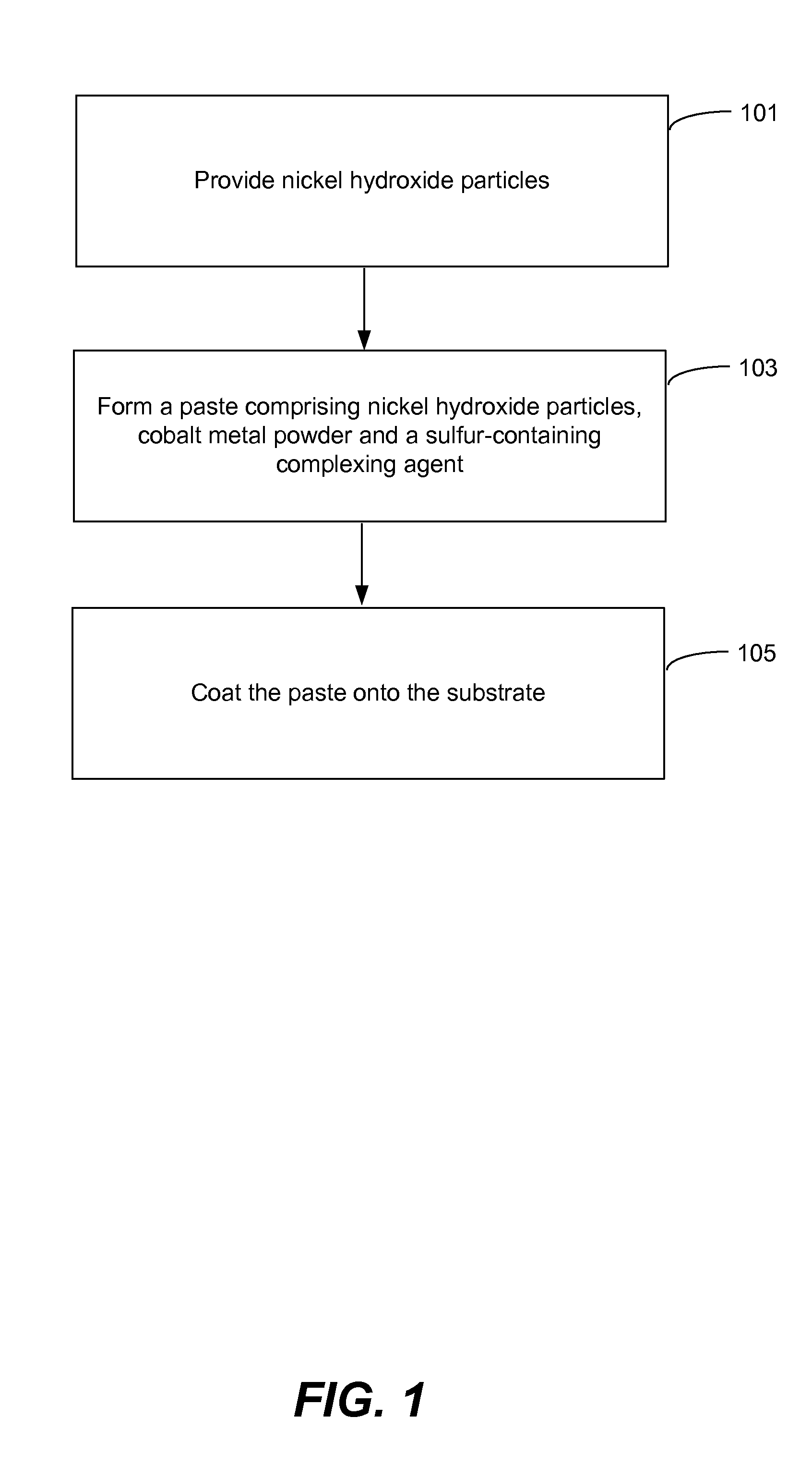
Pasted nickel hydroxide electrode and additives for rechargeable alkaline batteries
ActiveUS20140199591A1Prevent peelingMinimize migrationFinal product manufactureConductive materialNickel electrodeNickel zinc
A pasted positive nickel hydroxide electrode for use in battery cells (e.g., in nickel zinc cells, and nickel metal hydride cells) includes nickel hydroxide particles, a cobalt metal and / or cobalt compound and a sulfur-containing complexing agent capable of forming a complex with cobalt. The presence of the sulfur-containing complexing agent, such as dialkyldithiocarbamate (e.g., sodium diethyldithiocarbamate) improves lifetime and capacity utilization of the nickel electrode. The resulting pasted nickel hydroxide electrode includes a CoOOH conductive matrix after formation. The surface of the nickel hydroxide particles in the electrode is modified in some embodiments by providing a cobalt-containing coating onto the surface of the nickel hydroxide particles, followed by oxidation with a strong oxidizing agent. The complexing agent can be added before, after, or during the oxidation.
Owner:ZINCFIVE POWER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com