Method for preparing lithium-enriched solid solution cathode material by doping iron, copper and tin ions
A positive electrode material and solid solution technology, which is applied in the field of preparation of doped lithium-rich solid solution positive electrode materials, can solve the problems of poor electrochemical performance consistency of reaction products, uneven mixing of reactants, etc., and achieve excellent discharge performance, low raw material cost, and cycle good performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
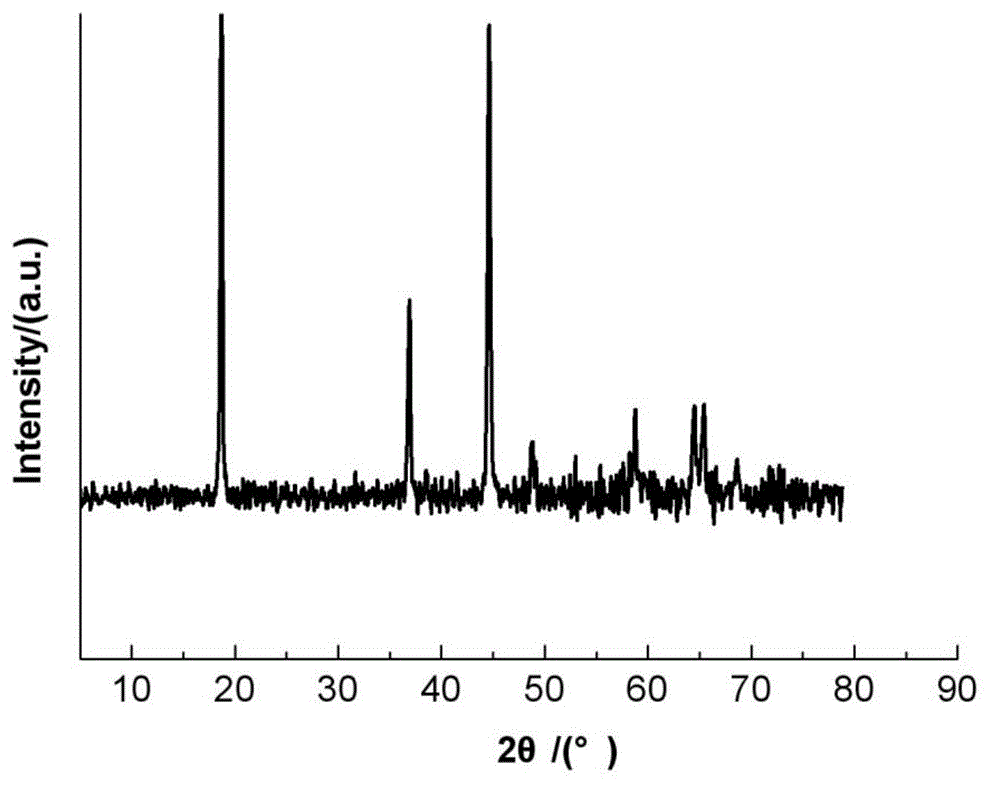
Image
Examples
Embodiment 1
[0032] Weigh lithium carbonate, nickel carbonate, manganese carbonate, cobalt carbonate, Fe 2 o 3 and formic acid.
[0033] Nickel carbonate, manganese carbonate, cobalt carbonate and Fe 2 o 3 Mix, add deionized water 15 times the volume of the total solid volume, add formic acid, wet mill and mix for 15 hours with an ordinary ball mill, then add lithium carbonate, wet mill and mix for 15 hours to obtain precursor 1; precursor 1 at 80 ° C, Precursor 2 was prepared by drying under a vacuum condition of 10 Pa. Precursor 2 was placed in a pure oxygen atmosphere, sintered at 550°C for 15 hours, and cooled to a room to obtain a precursor calcined material; the precursor calcined material was crushed and passed through a 200-mesh sieve, placed in an air atmosphere, and sintered at 1050°C sintering at lower temperature for 24 hours, the prepared composition is 0.30 Li 2 MnO 3 0.70 Li[Ni 0.30 mn 0.40 co 0.225 Fe 0.075 ]O 2 lithium-rich solid solution cathode material. When...
Embodiment 2
[0036] According to the molar ratio of lithium ion, nickel ion, manganese ion, cobalt ion, ferrous ion, oxalic acid is 1.57: 0.215: 0.742: 0.0344: 0.0086:1, weigh basic lithium carbonate, basic nickel carbonate, basic manganese carbonate , basic cobalt carbonate, FeO and oxalic acid.
[0037] Mix the weighed basic nickel carbonate, basic manganese carbonate, basic cobalt carbonate and FeO, add 1 / 10 times the volume of ethanol of the total volume of the solid, add glycine, and use a super energy ball mill to wet mix for 3 hours, then Add basic lithium carbonate, and use a super-energy ball mill to mix for 3 hours to obtain precursor 1; dry precursor 1 at 80° C. under a vacuum condition of 10132 Pa to prepare dry precursor 2. Precursor 2 was placed in an oxygen-enriched air gas atmosphere with an oxygen volume content of 22%, sintered at 300°C for 3 hours, and then placed in another sintering furnace with an air atmosphere, and sintered at 1050°C for 3 hours, the preparation com...
Embodiment 3
[0040] Weigh lithium hydroxide, nickel carbonate, manganese carbonate, basic cobalt carbonate, Cu (OH) 2 , formic acid.
[0041] Nickel carbonate, manganese carbonate, basic cobalt carbonate and Cu(OH) 2 Mix, add acetone 15 times the volume of the total solid volume, add formic acid, wet grind and mix for 3 hours with a wet grinder, then add lithium hydroxide, and mix with a wet grinder for 3 hours to obtain precursor 1; at 280 ° C Precursor 2 was prepared by drying in a vacuum at a pressure of 10132 Pa. Precursor 2 was placed in a pure oxygen atmosphere, sintered at 550 °C for 15 hours, and then placed in another sintering furnace with a pure oxygen atmosphere, and sintered at 800 °C for 24 hours to prepare a composition of 0.25 Li 2 MnO 3 0.75 Li[Ni 0.15 mn 0.40 co 0.30 Cu 0.15 ]O 2 lithium-rich solid solution cathode material. When the prepared sample is charged and discharged at 45°C, in the voltage range from 4.6 to 2.5V, and at a current of 0.8C, the discharge ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com