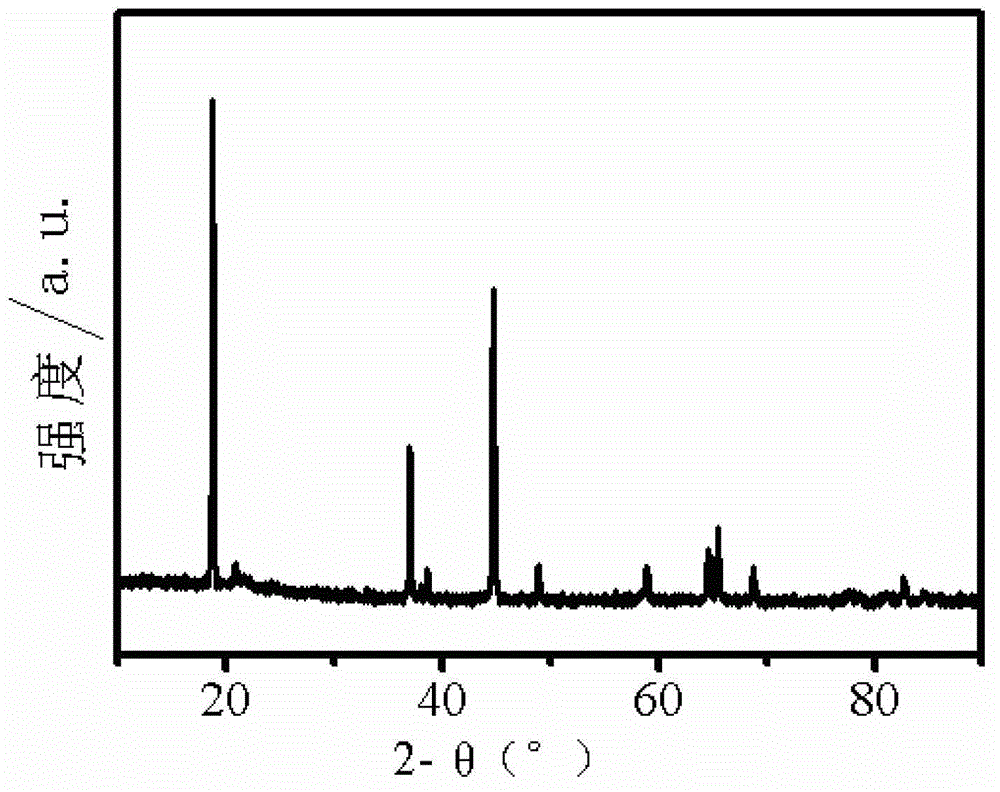
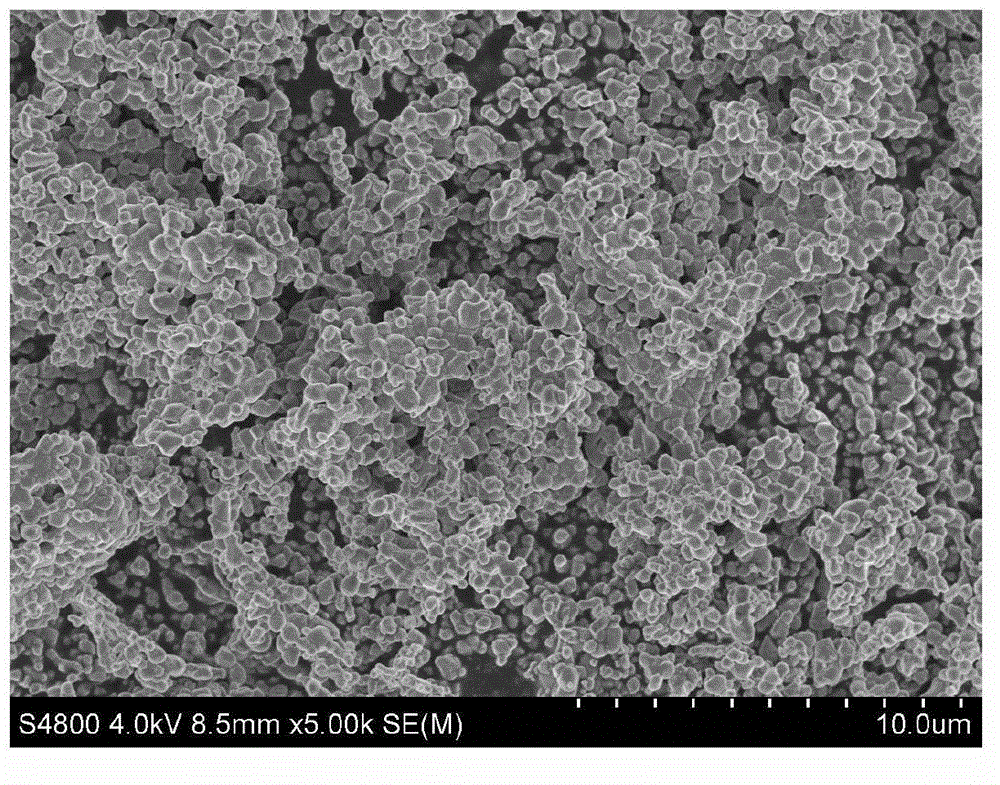
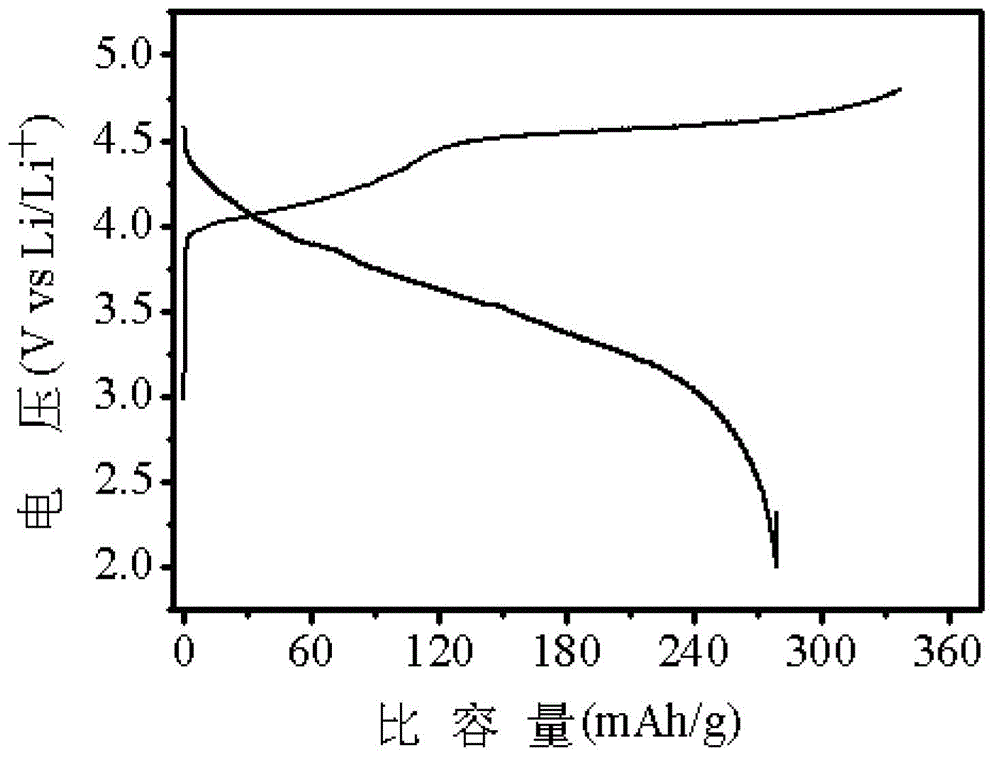
Lithium-rich manganese-based anode material and method for manufacturing same
A cathode material, lithium-rich manganese-based technology, applied in the field of new energy material preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0117] A preferred preparation method comprises the steps of:
[0118] Uniformly mix the lithium-rich manganese-based cathode material with a conductive agent and a binder in a solution (such as nitrogen-methylpyrrolidone (NMP)), and adjust the appropriate mass ratio of the lithium-rich manganese-based cathode material, conductive agent and binder (such as 85:10:5), and then coated and pressed on aluminum foil, and dried in a vacuum to obtain a positive electrode sheet.
[0119] Lithium-ion secondary battery
[0120] The lithium ion secondary battery provided by the invention comprises positive electrode material, negative electrode material, separator, electrolyte and shell. Wherein, the positive electrode material comprises the lithium-rich manganese-based positive electrode material Li[Li[Li] of the present invention x Ni a mn b m 1-a-b-x ]O 2The negative electrode material is natural graphite, artificial graphite, mesocarbon microspheres, silicon carbide, alloy mater...
Embodiment 1
[0128] 1.1. Weigh Lithium Acetate, Nickel Acetate, Cobalt Acetate and Manganese Acetate according to the molar ratio of 1.2:0.13:0.13:0.54, add them into deionized water and control the temperature at 30°C under magnetic stirring to fully dissolve them ;
[0129] 1.2. Then add resorcinol 2 times the amount of total metal ions and CTAB 1 / 20 times the amount of total metal ions; add hydrochloric acid catalyst, adjust the pH to 3.0, and stir magnetically at 30°C to dissolve and mix evenly; add Formaldehyde, according to the mol ratio of formaldehyde and resorcinol is the ratio of 2:1 to measure the formaldehyde solution, magnetically stirred for 30 minutes;
[0130] 1.3. Transfer the mixed solution obtained in 1.2 into a constant temperature drying oven and react at 80°C for 24 hours to obtain a pre-condensed polymer, and vacuum-dry the pre-condensed polymer at 120°C to obtain Interpreted condensate; the precalcined condensate is pre-calcined at 400°C for 5 hours in air, then he...
Embodiment 2
[0132] 2.1. According to the molar ratio of 1.2:0.17:0.07:0.56, weigh lithium acetate, nickel acetate, cobalt acetate and manganese acetate and add them into deionized water, and control the temperature at 80°C under magnetic stirring to fully dissolve;
[0133] 2.2. Then add resorcinol 1.5 times the amount of total metal ions and CTAB 1 / 30 times the amount of total metal ions; add oxalic acid catalyst to adjust the pH to 4.5, then magnetic stirring at 80°C to dissolve and mix evenly; Add formaldehyde, measure the formaldehyde solution according to the molar ratio of formaldehyde and resorcinol is 2:1, add to the above solution, and stir magnetically for 30 minutes;
[0134] 2.3. Transfer the mixed solution obtained in 2.2 into a constant temperature drying oven and react at 90°C for 48 hours to obtain a pre-condensed polymer, and then dry the pre-condensed polymer in vacuum at 130°C to obtain a pre-condensed product. The intercondensate is pre-calcined at 400°C for 5h in air,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com