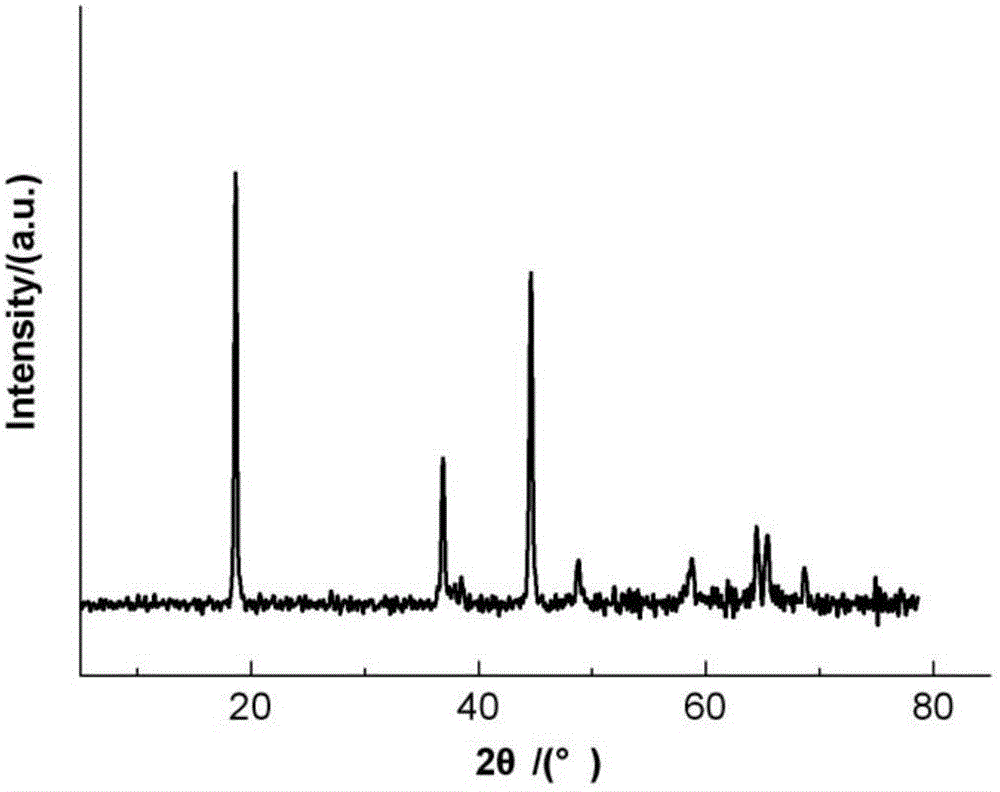
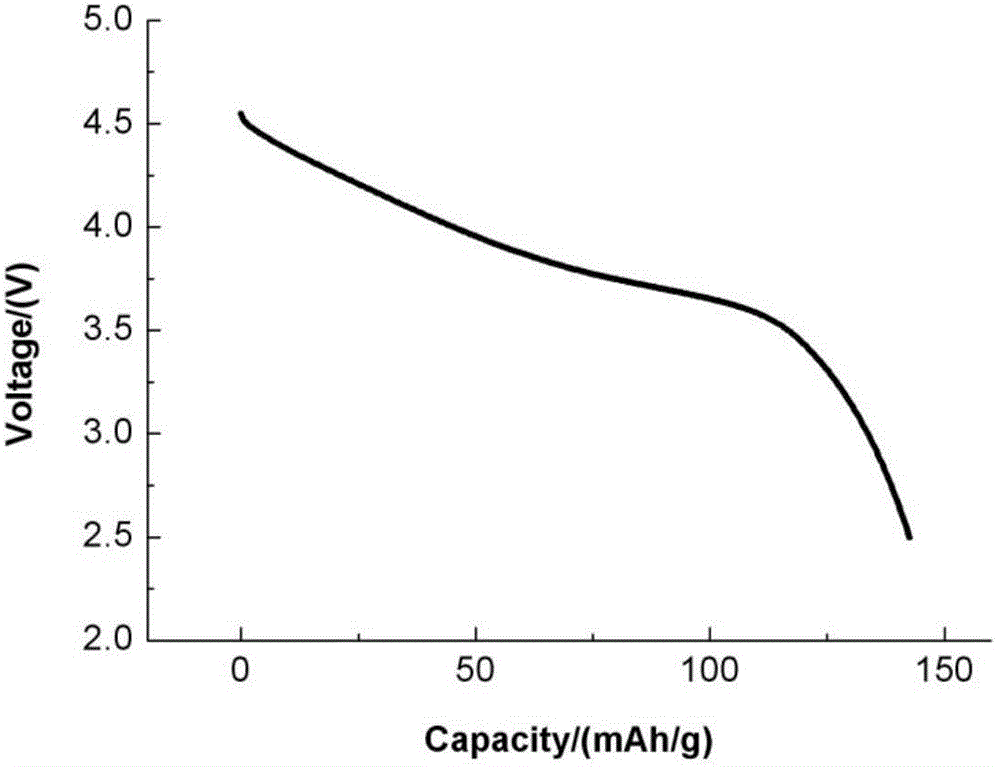
Method for preparing lithium-rich solid solution cathode material through reduction
A positive electrode material and solid solution technology, which is applied in the field of preparation of doped lithium-rich solid solution positive electrode materials, can solve problems such as the inability to eliminate the influence of dissolved oxygen in water, and achieve excellent discharge performance, good cycle performance, and less time-consuming effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Lithium hydroxide, nickel hydroxide, manganese acetate, cobalt hydroxide, and hydrazine were weighed according to the molar ratio of lithium ion, nickel ion, manganese ion, cobalt ion, and hydrazine being 1.35: 0.195: 0.526: 0.319: 0.27. The total weight of reactants is the total weight of lithium hydroxide, nickel hydroxide, manganese acetate, cobalt hydroxide and hydrazine. The weight ratio of the total weight of reactants to deionized water is 5:50 (equivalent to (2 · (1-x) · y + 4 · (x+z-x · z) + 3 · (1-x) · k-x-3) =0.0998)
[0037] Mix hydrazine with deionized water, then mix with weighed nickel hydroxide, manganese acetate, and cobalt hydroxide, wet mill and mix for 1 hour, add ammonia water to make the solution acidity pH 10, add weighed lithium hydroxide, wet mill and mix A reaction mixture solution containing a precipitate was prepared for 1 hour. The reaction mixture solution containing the precipitate was aged at 40°C for 2 hours to obtain precursor 1; the ...
Embodiment 2
[0040] According to the molar ratio of lithium ion, nickel ion, manganese ion, cobalt ion, vitamin C is 1.15: 0.0425: 0.358: 0.51: 0.01, respectively weigh lithium nitrate, nickel oxalate, manganese sulfate, cobalt chloride and vitamin C (equivalent to ( 2·(1-x)·y + 4·(x+z-x·z) + 3·(1-x)·k-x-3)=-0.10); the total reactants are lithium nitrate, nickel oxalate, manganese sulfate, For the total weight of cobalt chloride and vitamin C, weigh ethanol according to the total weight of reactants and ethanol weight ratio of 5:1.
[0041] Mix vitamin C with ethanol, then mix and weigh nickel oxalate, manganese sulfate and cobalt chloride, wet mill and mix for 15 hours, add ammonia water to make the solution acidity pH 13.5, add weighed lithium nitrate, wet mill and mix for 15 hours to prepare Precipitate in the reaction mixture solution. Precursor 1 was obtained by aging the reaction mixture solution containing the precipitate at 95° C. for 24 hours. Precursor 1 was dried at 110°C with...
Embodiment 3
[0044] According to lithium ion, nickel ion, manganese ion, cobalt ion, reducing agent molar ratio is 1.6: 0.18: 0.77: 0.02: 0.77 respectively weighs lithium iodide, nickel nitrate, manganese oxalate, cobalt oxalate, titanium trichloride; Reactant The total weight is the total weight of lithium iodide, nickel nitrate, manganese oxalate, cobalt oxalate and titanium trichloride, according to the total weight of reactants and methanol weight ratio is 1:1 weighing methanol (equivalent to (2 · (1-x ) y + 4 (x+z-x z) + 3 (1-x) k-x-3)=-0.10).
[0045] Mix titanium trichloride with methanol, then add the weighed nickel nitrate, manganese oxalate and cobalt oxalate, wet mill and mix for 10 hours, add ammonia water to make the solution acidity pH 10, add weighed lithium iodide, wet mill and mix for 11 h, prepare a reaction mixture solution containing the precipitate. Precursor 1 was obtained by aging the reaction mixture solution containing the precipitate at 40° C. for 24 hours. Prec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com