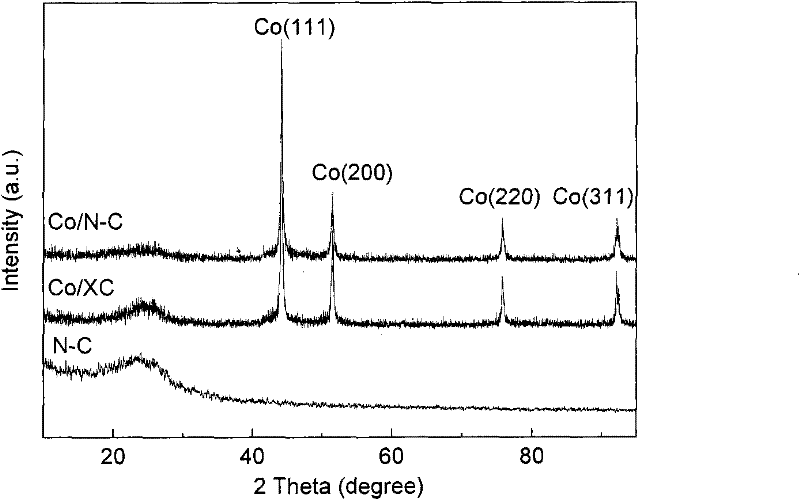
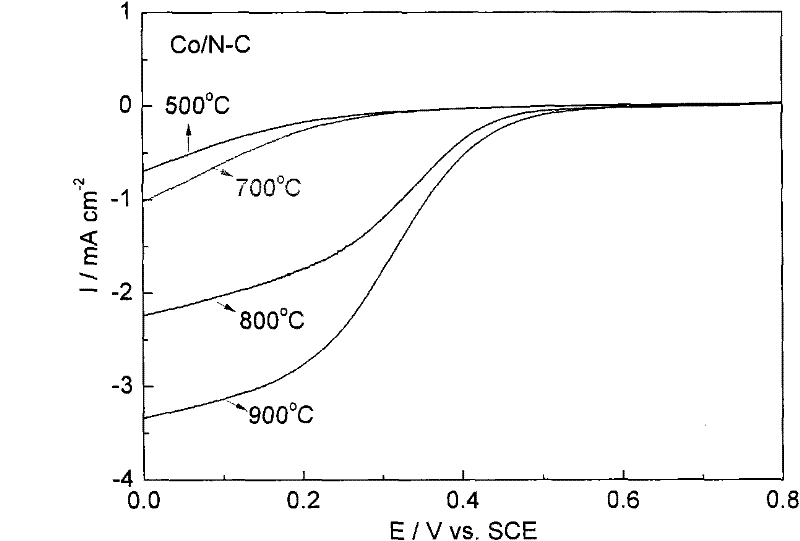
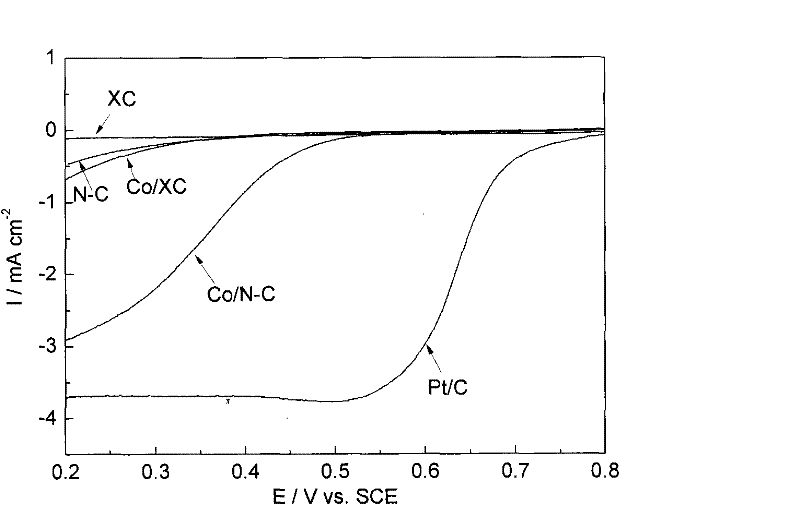
M/N-C catalyst and preparation and application thereof
A catalyst and oxidant technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problem of reducing the catalytic activity of the catalyst per unit mass and failing to form Co-N The problems of bit structure and poor conductivity of PPy can achieve the effects of enhanced conductivity, improved stability and strong conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] First, at a concentration of 0.8mol·L -1 Add pyrrole monomer in the cetyltrimethylammonium bromide (CTAB) ethylene glycol solution, the concentration of pyrrole monomer is 1mol L -1 , stirred at 0°C for 1 hour, then added the oxidant ferric chloride (FeCl 3 ), stirred at 0° C. for 2 hours to polymerize the pyrrole monomer to generate polypyrrole (PPy), and the molar ratio of ferric chloride to pyrrole monomer was 0.5:1;
[0030] Immerse the synthesized PPy into 100% electro-alcohol aqueous solution to remove residual surfactants and oxidants, then filter, wash, and dry under vacuum at 75°C for 3 hours;
[0031] Cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) added to a concentration of 2molL -1 In the ethylene glycol solution B of NaOH, the molar concentration of cobalt nitrate is 0.02mol L -1 , stirred at 180°C for 3 hours, then added the required amount of PPy, cobalt accounted for 30% of the total mass of cobalt and PPy, continued to stir for 2 hours, then added 40 times ...
Embodiment 2
[0035] First, at a concentration of 0.8mol·L -1 Add pyrrole monomer in the cetyltrimethylammonium bromide (CTAB) ethylene glycol solution, the concentration of pyrrole monomer is 1mol L -1 , stirred at 0°C for 1 hour, then added the oxidant ferric chloride (FeCl 3), stirred at 0° C. for 2 hours to polymerize the pyrrole monomer to generate polypyrrole (PPy), and the molar ratio of ferric chloride to pyrrole monomer was 0.5:1;
[0036] Immersing the synthesized PPy into 100% by mass methanol aqueous solution to remove residual surfactants and oxidants, then filtering, washing, and drying under vacuum at 75°C for 3 hours;
[0037] Cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) added to a concentration of 2molL -1 In the ethylene glycol solution of NaOH, the molar concentration of cobalt nitrate is 0.02mol L -1 , then stirred at 180°C for 3 hours, then added the required amount of PPy, cobalt accounted for 30% of the total mass of cobalt and PPy, continued to stir for 2 hours, then a...
Embodiment 3
[0041] First, at a concentration of 0.8mol·L -1 Add pyrrole monomer in the cetyltrimethylammonium bromide (CTAB) ethylene glycol solution, the concentration of pyrrole monomer is 1mol L -1 , stirred at 0°C for 1 hour, then added the oxidant ferric chloride (FeCl 3 ), stirred at 20°C for 2 hours to polymerize the pyrrole monomer to generate polypyrrole (PPy), and the molar ratio of ferric chloride to pyrrole monomer was 0.5:1;
[0042] Immersing the synthesized PPy into 100% by mass methanol aqueous solution to remove residual surfactants and oxidants, then filtering, washing, and drying under vacuum at 75°C for 3 hours;
[0043] Cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) added to a concentration of 2molL -1 In the ethylene glycol solution of NaOH, the molar concentration of cobalt nitrate is 0.02mol L -1 , then stirred at 180°C for 3 hours, then added the required amount of PPy, cobalt accounted for 30% of the total mass of cobalt and PPy, continued to stir for 2 hours, then a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com