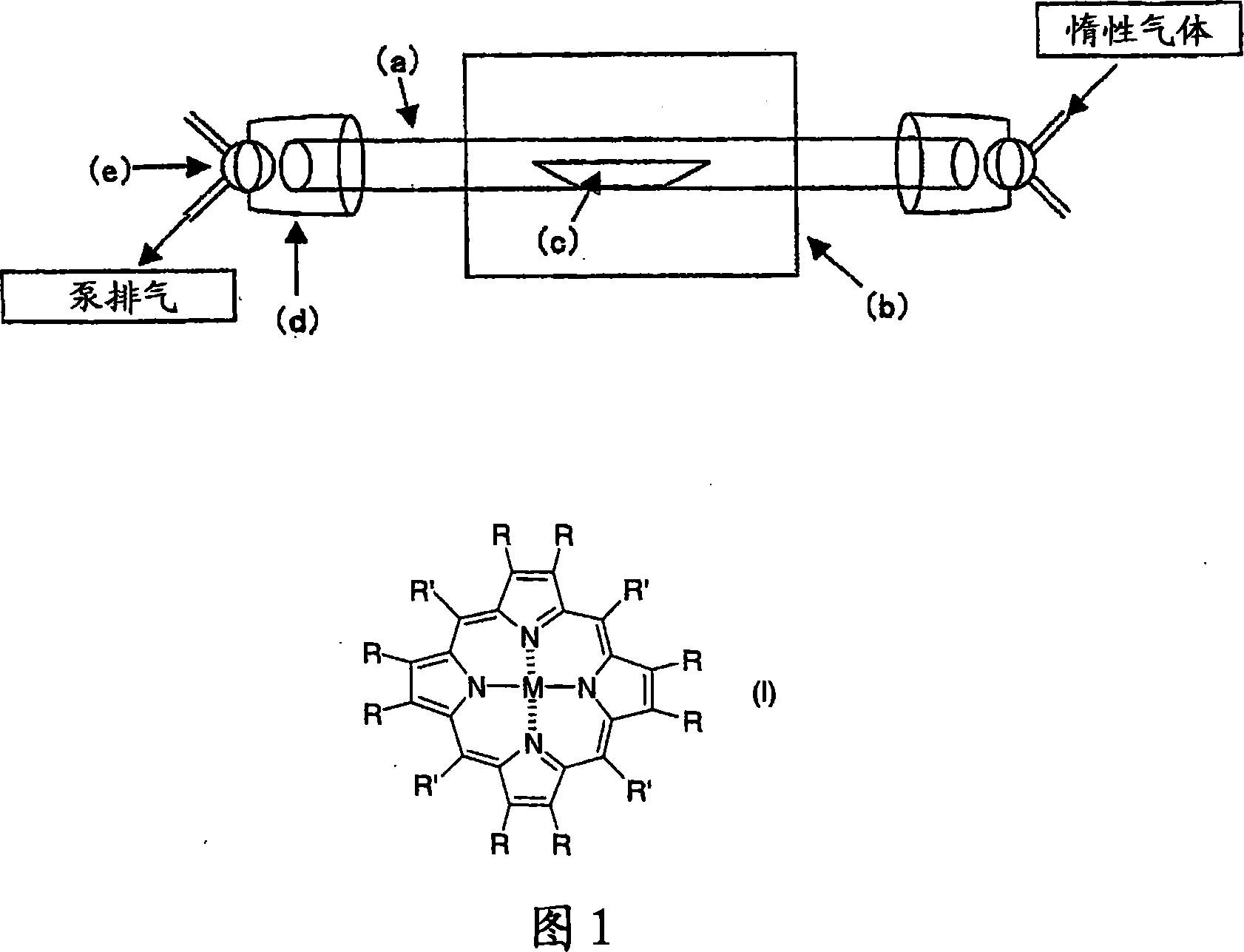
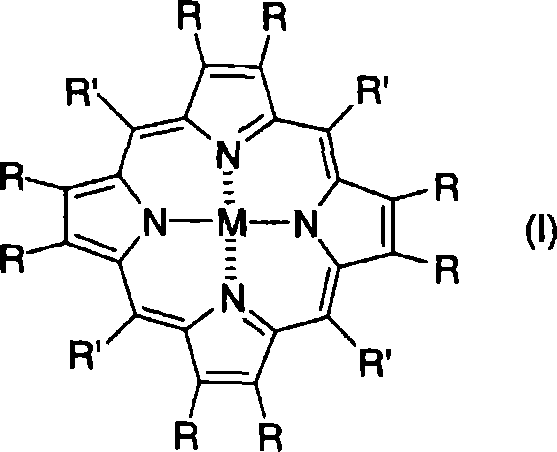
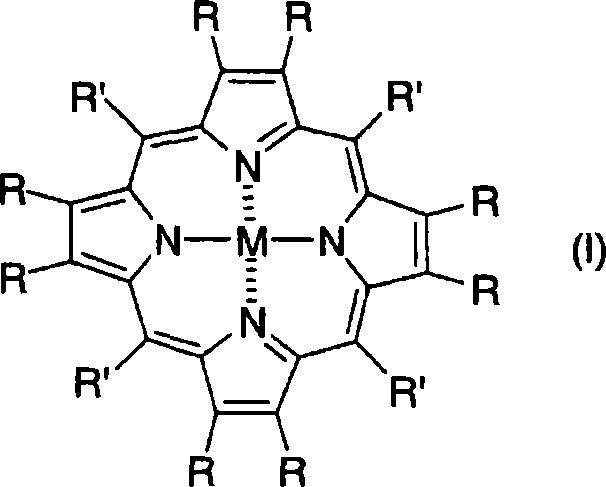
Porphyrin-based electrode catalyst
A catalyst and porphyrin technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, battery electrodes, etc., can solve the problems of low oxygen reduction activity, unfavorable induction of 2-electron reduction, catalysts that cannot be put into practical use, etc., and achieve high The effect of oxygen reduction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the synthesis of 5,10,15,20-tetra(3-thienyl)porphyrin
[0062]
[0063] A porphyrin in which all 4 meso positions were substituted with 3-thienyl was synthesized.
[0064] Propionic acid (200ml) was added to a 2-L four-necked flask to heat it to 140°C, and pyrrole (5.6ml, 81mmol) and 3-thienaldehyde (7.0ml, 80mmol) were added thereto. After the reaction was completed, the reaction solution was cooled, cold methanol was added for suction filtration, the residue was dissolved in chloroform, and the resulting substance was washed twice with water, aqueous sodium hydroxide solution and water. The organic layer was dried over magnesium sulfate, and the solvent was removed by distillation. The residue was eluted with chloroform by column chromatography on silica gel (5 cm()×50 cm), fractions containing the target product were collected, the solvent was removed by distillation, and the resulting crystals were recrystallized from chloroform / hexane to obtain ...
Embodiment 2
[0067] Example 2: Synthesis of 5,10,15,20-tetra(3-thienyl)porphyrin cobalt complex (CotthP)
[0068]
[0069] The cobalt complex of tetrakis(3-thienyl)porphyrin obtained in Example 1 was synthesized.
[0070] DMF (100 ml) and 300 mg of the tetrakis(3-thienyl)porphyrin obtained in Example 1 were added and dissolved in a 500 ml round bottom flask, and the resultant was degassed with argon.
[0071] Cobalt acetate tetrahydrate (585 mg) was sonicated therein and the resulting mass was heated at 150°C to 160°C under reflux for 2 hours using a Dimroth reflux condenser equipped with an argon balloon. After the reaction was completed, the resulting material was ice-cooled to 4° C. or lower, and an excess of ice-cooled water was added for recrystallization (DMF / water). Crystals were recovered by suction filtration using a glass filter, and then dried in vacuo (120° C., 6 hours) to obtain the title compound (CotthP) (267 mg, 82%). The product was identified by UV analysis (UV-210...
Embodiment 3
[0072] Example 3: Carrying the porphyrin complex on a conductive carrier (carbon)
[0073] CotthP obtained in Example 2 was used as a porphyrin complex. Carbon black (Ketjen Black) was used as the conductive carrier.
[0074] Carbon black (500 mg) was ultrasonically dispersed in chloroform. The dispersion liquid is stirred at room temperature to 58° C. for 1.5 hours using a magnetic stirrer, a high shear stress type stirrer, or the like. CotthP was added using a syringe and the mixture was stirred while cooling to 30°C for 3-6 hours. After the stirring was completed, chloroform was removed by distillation and the residue was dried in vacuo to obtain a porphyrin complex-supporting carbon.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com