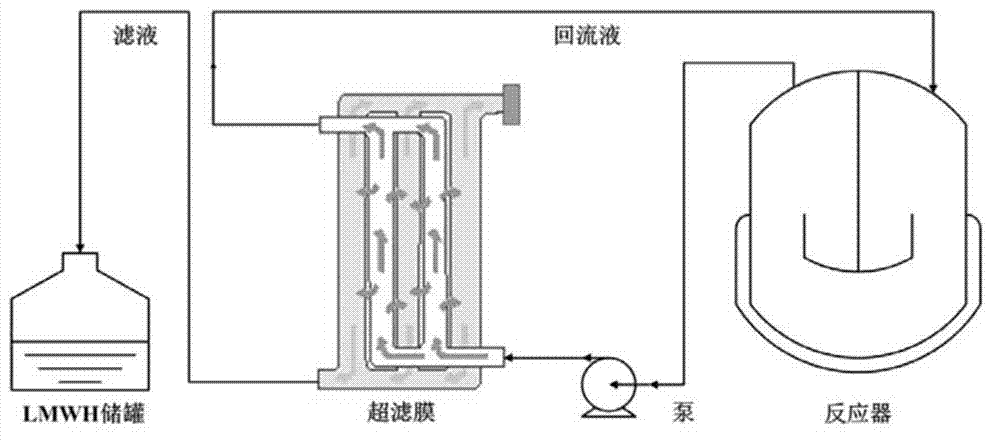
Method for controlling production of low-molecular-weight heparin
A low molecular weight heparin, low molecular weight technology, applied in the field of controlled production of low molecular weight heparin or ultra-low molecular weight heparin, can solve problems such as limiting the application of heparin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] The (ultra) low molecular weight heparin product obtained in Example 1 adopts high performance exclusion chromatography to measure its average molecular weight (including the weight average molecular weight M w and number average molecular weight M n ) and distribution. The gel chromatography column used is TSK Gel G3000SW, 30mm×750mm, Tosoh Co., Japan. The HPLC system used includes computer control system, peristaltic pump (LC-10ATvp), autosampler (SIL-10ADvp), ultraviolet detector (SPD-M10Avp), differential detector (RID-10A). Wherein the ultraviolet detector and the differential detector are connected in series, and the differential detector is connected behind the ultraviolet detector. The mobile phase used was pH 5.0, containing 2.84% Na by mass percentage 2 SO 4 buffer. The column temperature is 35°C, the flow rate is 0.5mL / min, and the detection wavelength of the ultraviolet detector is 235nm.
[0113] (Ultra) low molecular weight heparin molecular weight s...
Embodiment 2
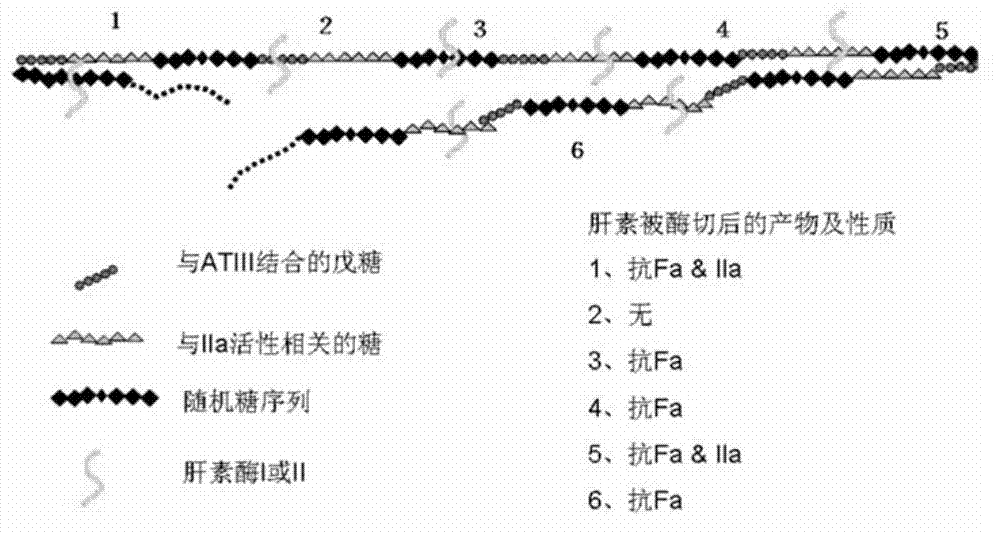
[0142] Example 2 Comparison of heparinase I and heparinase III degrading heparin
[0143] Add equal amounts of heparinase I and heparinase III to the heparin solution, degrade completely at 30°C, and react until A 235 No change occurs. The product was then passed through a membrane, precipitated and evaporated to dryness under reduced pressure. The products after evaporation to dryness were subjected to molecular weight identification and anti-Xa, IIa factor activity detection, and the results are shown in Table 2. Among them, the 1# sample is the product obtained by the degradation of heparinase I, with low molecular weight and low anti-Xa and anti-IIa activities, indicating that heparanase I can cut the anticoagulant active center structure of heparin. With the prolongation of cutting time, Most of the active centers are destroyed; 2# sample is the degradation product of heparanase III, which has a larger molecular weight than 1# sample, and the important thing is that t...
Embodiment 3
[0147] Example 3 Comparison of heparinase III and heparanase III, I combined use to degrade heparin
[0148] In order to test the differences in the action sites of heparanase I and heparanase III, the following experiments were designed. First use heparanase III to degrade heparin, degrade it completely at 30°C, and react until A 235 No change occurs, then take out half of the solution, then add heparanase I to it for cleavage, keep the other half of the solution as a control, and finally evaporate the two samples to dryness under reduced pressure. The products after evaporation to dryness were subjected to molecular weight identification and anti-Xa, IIa factor activity detection, and the results are shown in Table 3. Among them, the 2# sample is the product obtained by the degradation of heparanase III (see Example 2), with a molecular weight of 8299; the 3# sample is firstly degraded completely by using heparanase III under the same conditions as in Example 5 at 30°C ,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com