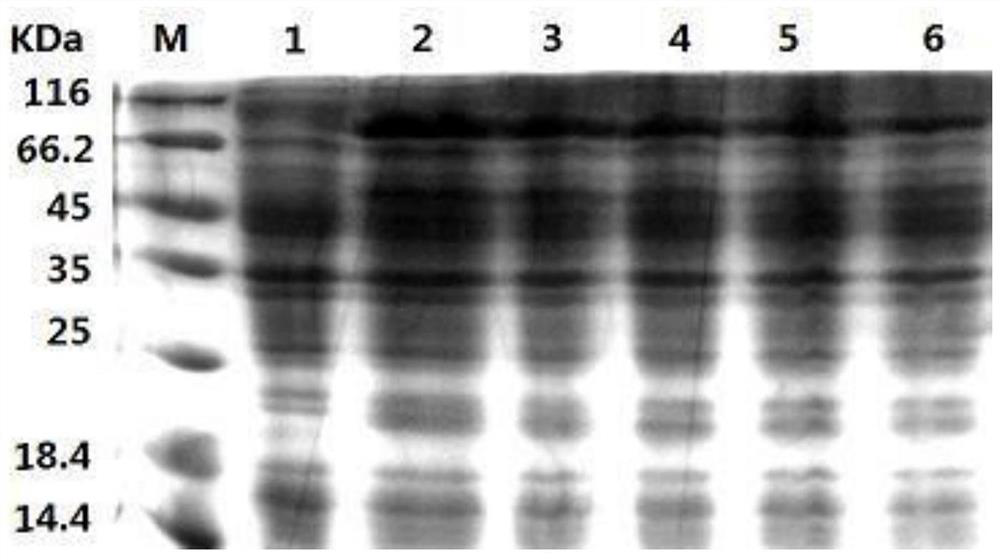
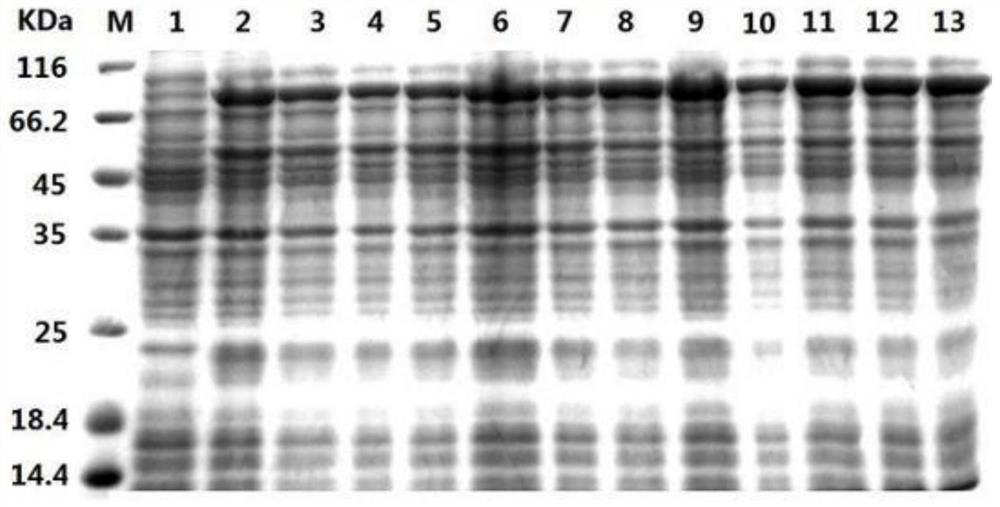
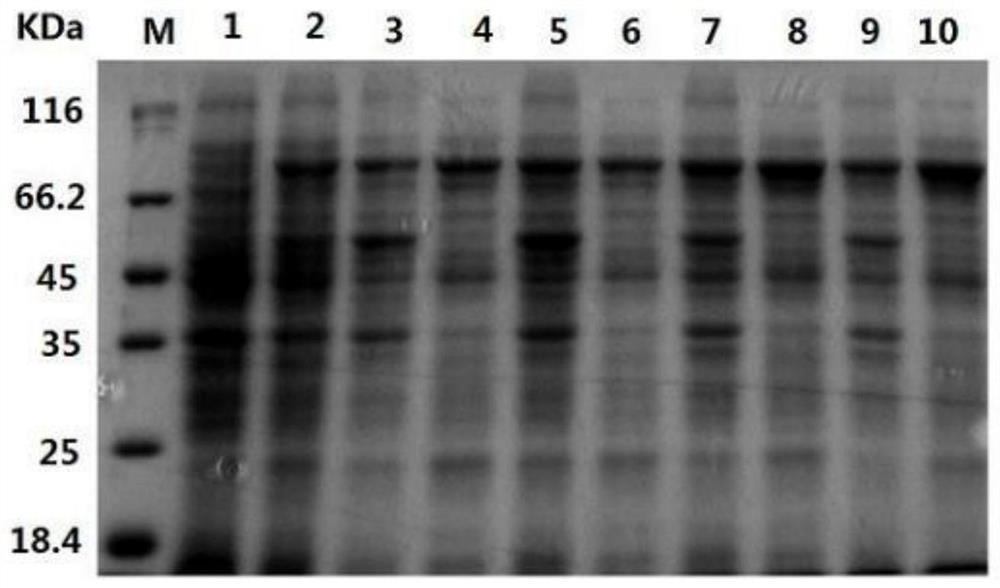
Method for preparing recombinant heparinase III by utilizing SUMO fusion expression system and SUMO_heparinase III fusion protein prepared by method
A fusion protein and fusion expression technology, applied in the biological field, can solve the problems of reducing the yield of the target protein and interfering with the normal function of the target protein, and achieve the effects of improving stability and solubility, promoting correct folding, and improving purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. Plasmid transformation into BL21(DE3) competent cells
[0056] 1.1 Add 2 μL of the plasmid to 100 μL of competent bacteria and place on ice for 30 minutes;
[0057] 1.2 Heat shock at 42°C for 90s, quickly place on ice for 5min; add 500μL LB culture solution; 1.3 Shake at 37°C, 220rpm for 1h, centrifuge and spread all on the resistant LB plate, and incubate overnight at 37°C.
[0058] 2. Expression Identification
[0059] 2.1 Pick 5 single clones on the plate and inoculate them in a test tube containing 4mL LB culture medium with an appropriate amount of resistance;
[0060] 2.2 Shake at 220 rpm at 37°C until the OD600 of the bacteria is 0.6-0.8;
[0061] 2.3 Take out 1 mL of the culture, centrifuge at 12000 g for 5 min at room temperature, discard the supernatant, resuspend the bacterial pellet with 80 μL of 1×PBS buffer and add 20 μL of 5×Loading Buffer;
[0062] 2.4 Add IPTG to the remaining culture to a final concentration of 0.5 mM, shake at 220 rpm at 37°C fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com