Thromboelastography TEG quality control and preparation method thereof
A technology of thromboelastography and quality control products, which is applied in the field of blood coagulation detection, can solve the problems of high biological safety risk, unfavorable scale production, and small quantity, and achieve high detection quality and improve the effect of quality detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
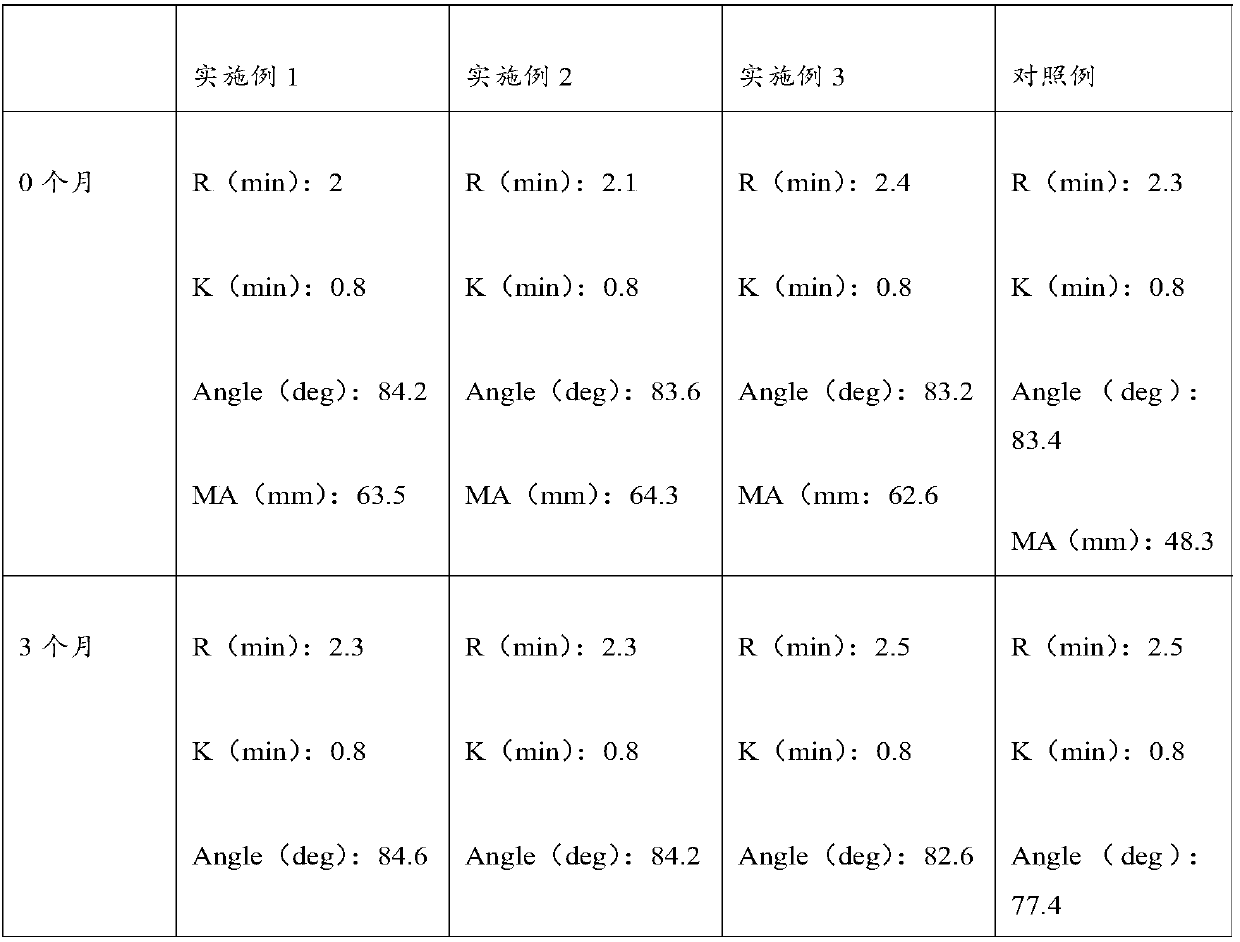
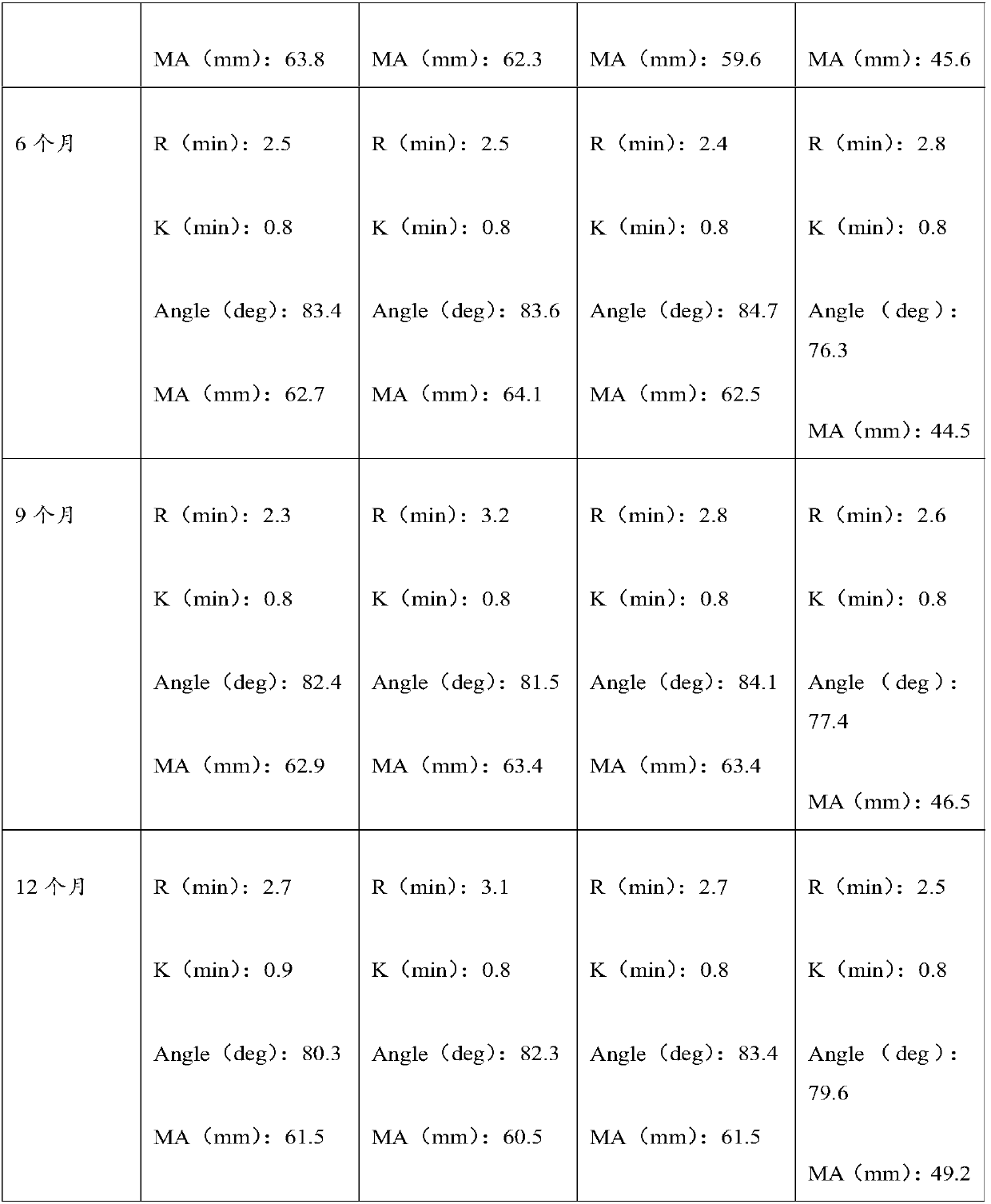
Embodiment 1
[0035] The preparation method of the thromboelastography quality control product provided by the present embodiment comprises the following steps:
[0036] Preparation steps of anticoagulant:
[0037] According to the ratio of parts by mass, 3 parts of sodium citrate, 7 parts of sucrose, 3 parts of sodium chloride, 0.01 part of mercury sulfide and 0.5 part of sodium azide were mixed.
[0038] Mixing steps:
[0039] The sodium citrate anticoagulant was mixed with the collected animal blood in a volume ratio of 1:9. Wherein, the animal plasma is porcine plasma.
[0040] Standing steps:
[0041] The mixed animal plasma was allowed to stand at room temperature until the layers were separated, the standing time was 10 h, and the room temperature was 15°C.
[0042] Compared with centrifuged plasma, the use of natural sedimentation can protect coagulation components such as coagulation factors, coagulation factors, fibrinogen, and platelets from being lost or activated.
[0043]...
Embodiment 2
[0050] The preparation method of the thromboelastography quality control product provided in this example is roughly the same as the preparation method provided in Example 1, the difference lies in the different parameters, and the difference is as follows:
[0051] Preparation steps of anticoagulant:
[0052] According to the ratio of parts by mass, 4 parts of sodium citrate, 8 parts of sucrose, 3 parts of sodium chloride, 0.01 part of mercury sulfide and 0.5 part of sodium azide were mixed.
[0053] Mixing steps:
[0054] The sodium citrate anticoagulant was mixed with the collected animal blood in a volume ratio of 1:9. Wherein, the animal plasma is porcine plasma.
[0055] Standing steps:
[0056] The mixed animal plasma was allowed to stand at room temperature until layers were separated, the standing time was 12 hours, and the room temperature was 15°C.
[0057] Extraction steps:
[0058] Extract the platelet-rich animal plasma (supernatant plasma) after standing an...
Embodiment 3
[0064] The preparation method of the thromboelastography quality control product provided in this example is roughly the same as the preparation method provided in Examples 1 and 2, the difference lies in the parameters, which are as follows:
[0065] Preparation steps of anticoagulant:
[0066] According to the ratio of parts by mass, 5 parts of sodium citrate, 10 parts of sucrose, 3 parts of sodium chloride, 0.01 part of mercury sulfide and 0.5 part of sodium azide were mixed.
[0067] Mixing steps:
[0068] The sodium citrate anticoagulant was mixed with the collected animal blood in a volume ratio of 1:9. Wherein, the animal plasma is porcine plasma.
[0069] Standing steps:
[0070] The mixed animal plasma was allowed to stand at room temperature until layers were separated, the standing time was 11 h, and the room temperature was 20°C.
[0071] Extraction steps:
[0072] Extract the platelet-rich animal plasma (supernatant plasma) after standing and stratifying, and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com