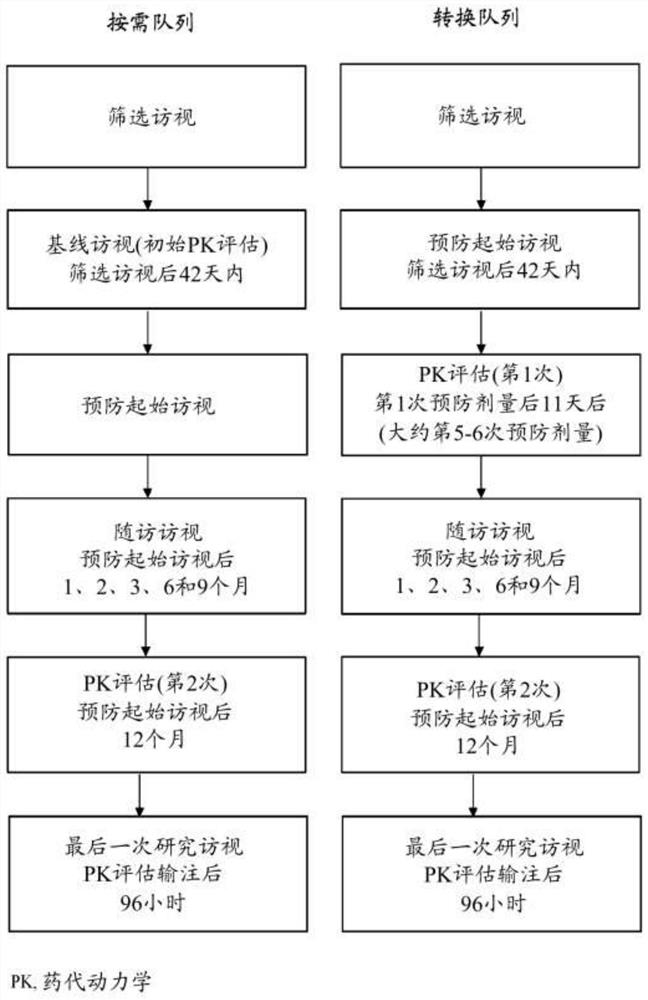
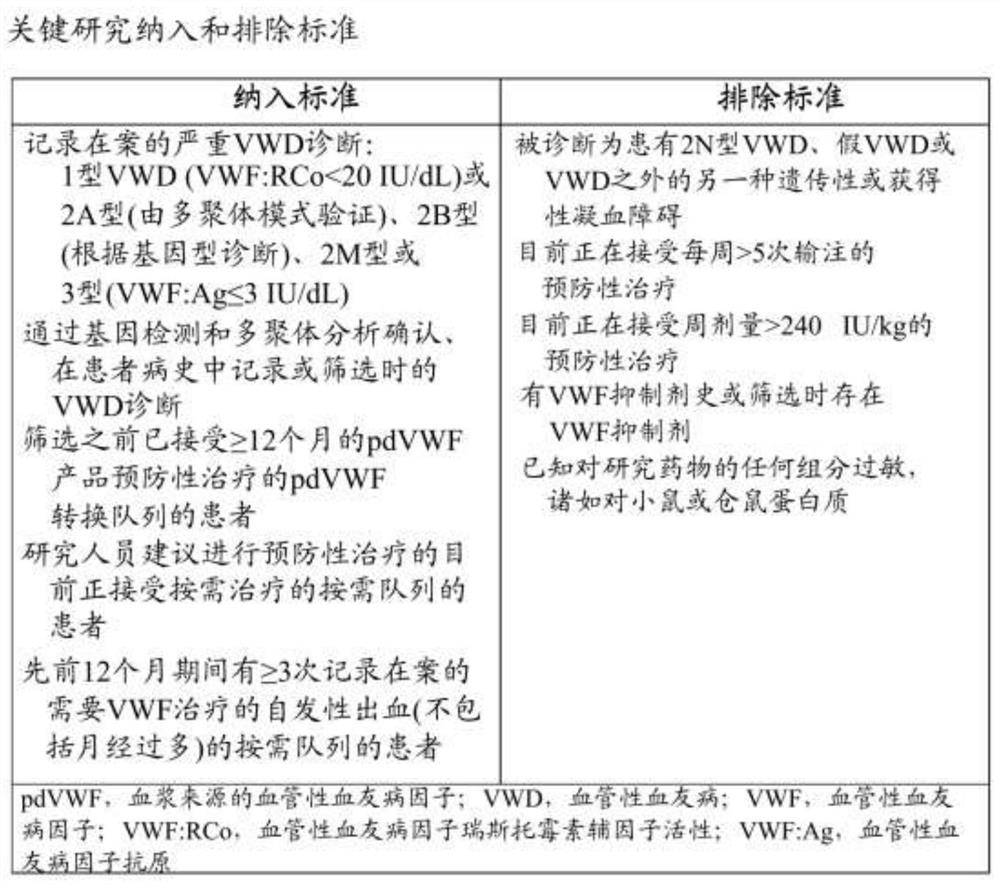
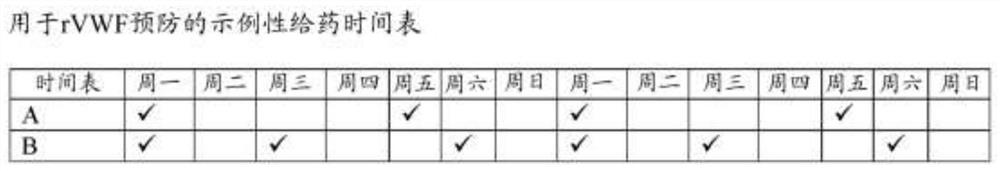
Methods of prophylactic treatment using recombinant vwf (RVWF)
A preventive, serious technology, applied in the direction of chemical instruments and methods, drug combinations, pharmaceutical formulations, etc., can solve problems such as preventive treatment is not well described or understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0254] k. Preparation methods of pharmaceutical preparations
[0255] The present invention also contemplates methods for the preparation of pharmaceutical formulations.
[0256] The method also includes one or more of the following steps: adding a stabilizer as described herein to the mixture prior to lyophilization; adding at least one selected from bulking agents, osmotic pressure regulators and Agents of surfactants, each as described herein, are added to the mixture.
[0257] Standard reconstitution practice for lyophilized material is to add back a volume of purified water or sterile water for injection (WFI) (usually equivalent to the volume removed during lyophilization), only in the preparation of drugs for parenteral administration Dilute solutions of antibacterial agents are sometimes used (Chen, Drug Development and Industrial Pharmacy, 18:1311-1354 (1992)). Accordingly, there is provided a method for preparing a reconstituted rVWF composition comprising the st...
Embodiment 1
[0316] Example 1: Efficacy and Safety of Recombinant Von Willebrand Factor (VWF) Prophylaxis in Severe Von Willebrand Disease (VWD): Design of a Prospective, Phase 3, Open-Label, International Multicenter Study
[0317] introduction
[0318] Patients with von Willebrand disease (VWD) have impaired hemostasis due to quantitative or qualitative defects in von Willebrand factor (VWF), a large multimeric plasma glycoprotein, It has key hemostatic functions that mediate platelet aggregation and stabilize circulating coagulation factor VIII (FVIII) (1-3). Most patients with VWD experience mild to moderate mucosal bleeding and hemorrhage after trauma or surgery, but life-threatening bleeding can also occur, especially in patients with severe disease (1,2).
[0319] Recombinant VWF (rVWF, vonicog alfa, VEYVONDI TM Baxalta Innovations GmbH, a Takedacompany, Vienna, Austria) was manufactured by recombinant DNA technology in Chinese hamster ovary cell lines without the addition of fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com