Patents
Literature
71 results about "Haemophilia A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
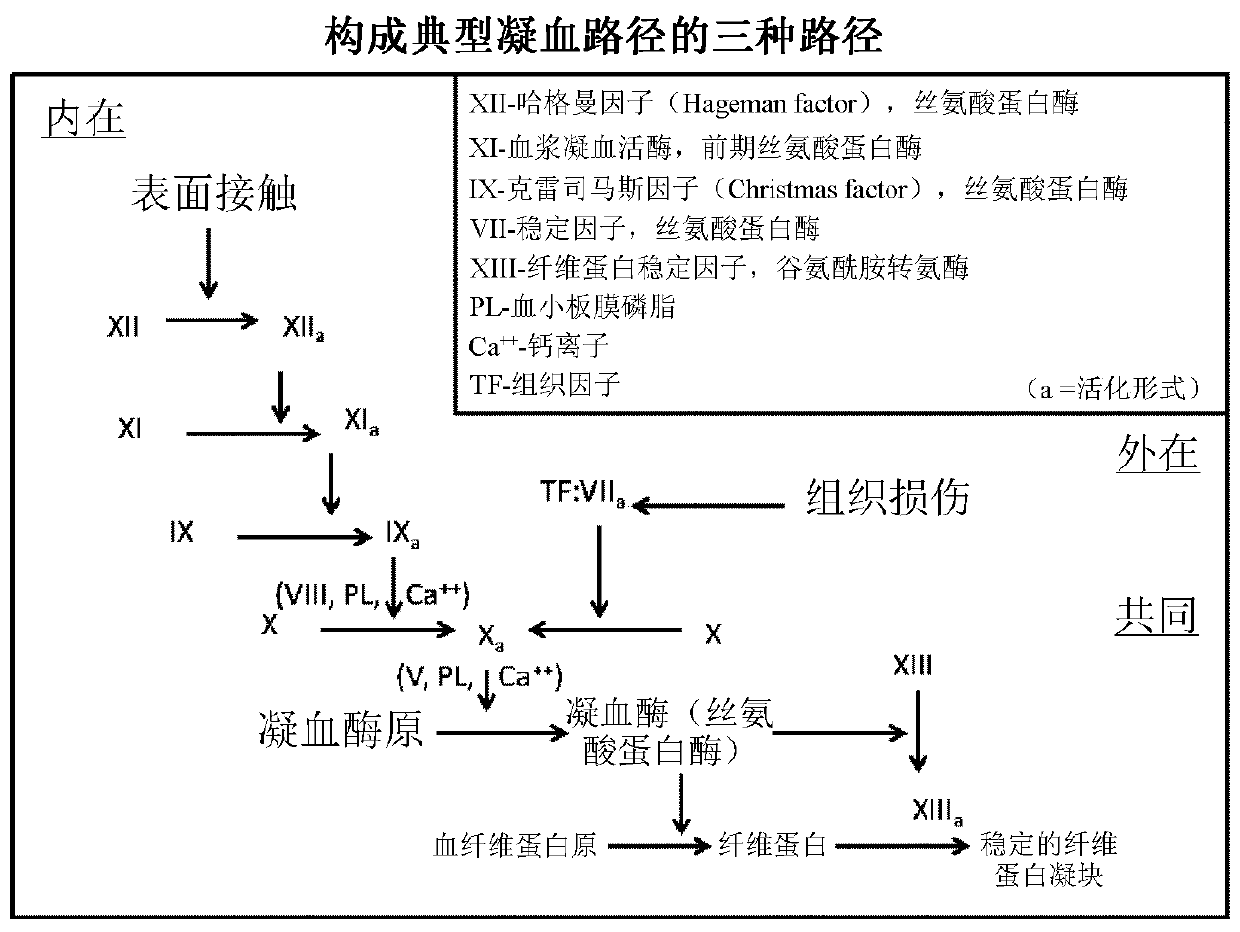
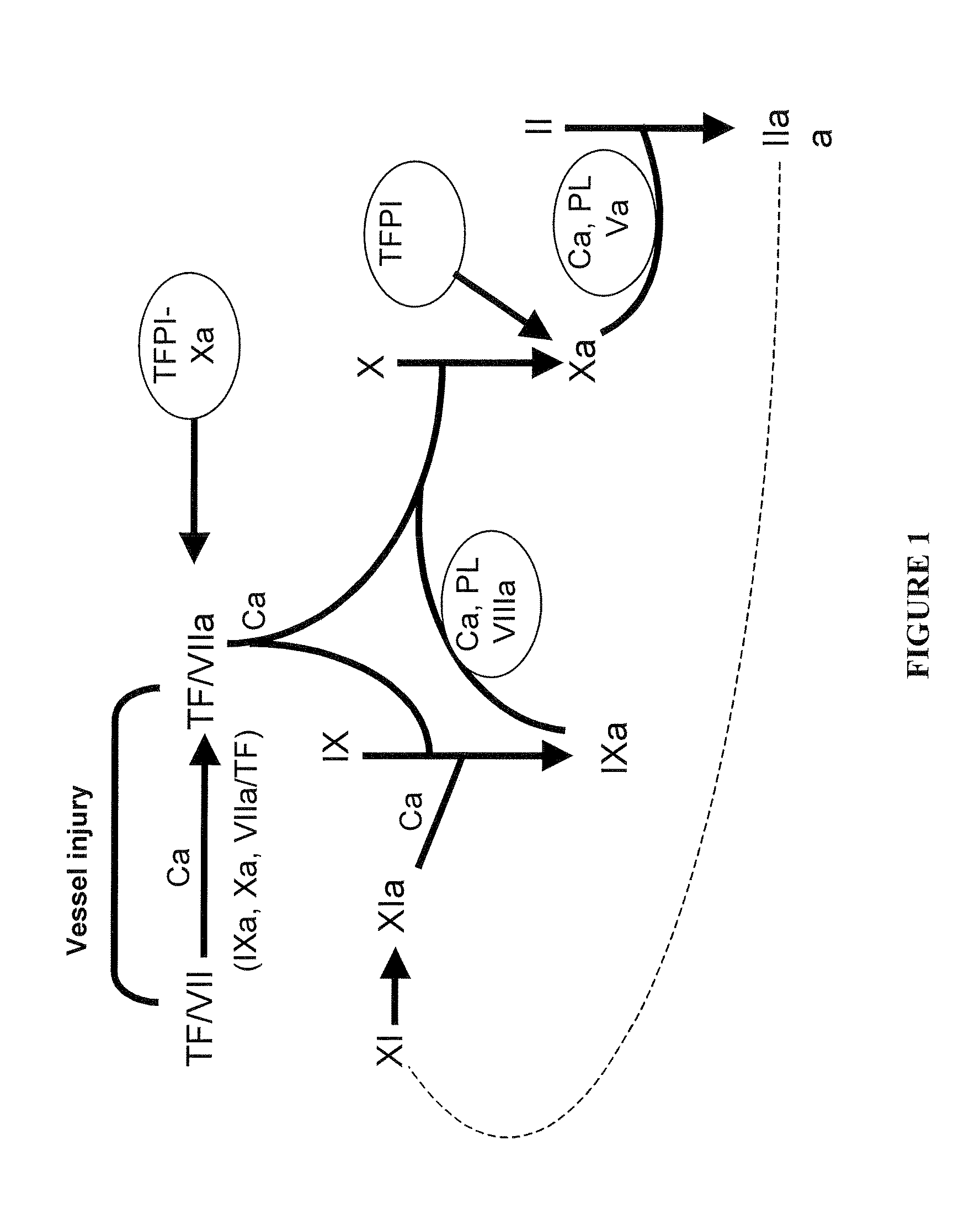
Haemophilia A (or hemophilia A) is a genetic deficiency in clotting factor VIII, which causes increased bleeding and usually affects males. In the majority of cases it is inherited as an X-linked recessive trait, though there are cases which arise from spontaneous mutations.
Recombinant factor VIII having reduced inactivation by activated protein C
ActiveUS8183345B2High catalytic efficiencyPromote localizationFactor VIIBacteriaProtein activationClotting disorders
The present invention relates to a recombinant factor VIII that is characterized by one or more mutations within a region surrounding an activated protein C cleavage site, which one or more mutations result in a reduced rate of inactivation by activated protein C. Isolated nucleic acid molecules, recombinant expression vectors, and host cells suitable for expression of the recombinant factor VIII are also disclosed. The recombinant factor VIII can be used for the treatment of clotting disorders, such as hemophilia A.
Owner:UNIVERSITY OF ROCHESTER
Method of providing hemostasis in Anti-coagulated blood
InactiveUS20080254147A1Promote formationBiocideInanimate material medical ingredientsMedicineB hemophilia
In a method of clotting blood in which the blood exhibits a reduced tendency to clot and may be from a person undergoing an anticoagulant therapy or having type A or B hemophilia or von Willebrand disease, a therapeutically effective amount of a composition comprising clay as the active ingredient is administered to a wound from which the blood emanates. Upon contacting the blood, this clay, which may be kaolin, bentonite, or any type of layered clay, causes the blood to clot. In a method of arresting blood flowing from a wound, a therapeutically effective amount of a composition comprising clay as the active ingredient is administered to the bleeding wound. In this method, the blood has a reduced tendency to clot and may be from a person undergoing an anticoagulant therapy or having at least one of hemophilia A or B or von Willebrand disease.
Owner:TELEFLEX LIFE SCI LTD
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
ActiveUS6887852B1Same pharmaceutical efficacyAvoid virus infectionFactor VIIPowder deliveryMedicineArginine
Disclosed is a lyophilized preparation of recombinant factor VIII used as a therapeutic preparation of hemophilia A. The lyophilized preparation of recombinant factor VIII is prepared by performing lyophilization using a mixture comprising 6 to 100 mM of L-arginine, 3.5 to 50 mM of L-isoleucine, and 10 to 100 mM of L-glutamic acid as a stabilizer for stabilizing the recombinant factor VIII which exhibits an unstable activity during lyophilization, rather than using human blood derived albumin.
Owner:KOREA GREEN CROSS CORP
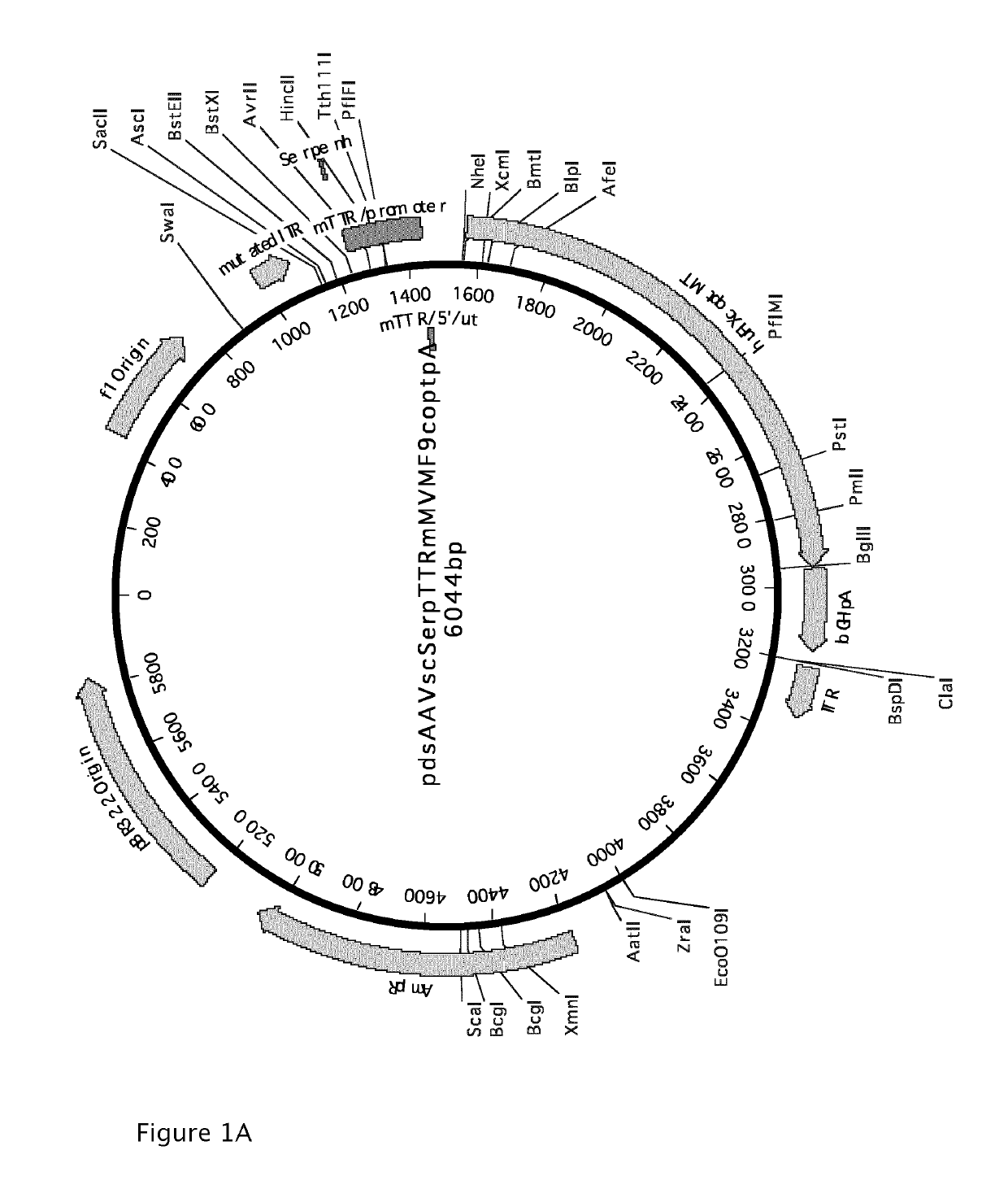
High capacity recombinant adenoviral vector for treatment of hemophilia A
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
Therapeutic method for blood coagulation disorder
InactiveUS20090148425A1Ensure adequate treatmentImprove abilitiesBiocidePeptide/protein ingredientsFactor iiPlatelet
The present invention provides agents for treating blood coagulation abnormalities, which contain as an active ingredient a lentiviral vector carrying a blood coagulation factor gene operably linked to a promoter which induces platelet-specific expression. Agents for treating hemophilia A or hemophilia B are provided by application of the gene encoding Factor VIII or Factor IX. Blood coagulation abnormalities can be treated by gene therapy by infecting hematopoietic stem cells or such with the therapeutic agents of the present invention.
Owner:DNAVEC CORP
Factor viii sequences
ActiveUS20150158930A1Many symptomIncrease of functional factor VIIIFactor VIIBacteriaGene deliveryNucleotide
There is provided a nucleic acid molecule comprising a nucleotide sequence encoding for a functional factor VIII protein, wherein the portion of the nucleotide sequence encoding for the B domain of the factor VIII protein is between 90 and 111 nucleotides in length and encodes for an amino acid sequence comprising a sequence having at least 85% identity to SEQ ID NO: 4 and which comprises six asparagine residues. Also provided is a functional factor VIII protein, a vector comprising the above nucleic acid molecule, a host cell, a transgenic animal, a method of treating haemophilia, e.g. haemophilia A, and a method for the preparation of a parvoviral gene delivery vector.
Owner:UCL BUSINESS PLC
Recombinant factor viii having enhanced stability following mutation at the a1-c2 domain interface
InactiveUS20120065136A1Enhance inter-domain binding affinityIncreased stability parameterFactor VIIFungiFactor iiHaemophilia A
The invention relates to a recombinant factor VIII that includes one or more mutations at an interface of A1 and C2 domains of recombinant factor VIII. The one or more mutations include substitution of one or more amino acid residues with either a cysteine or an amino acid residue having a higher hydrophobicity. This results in enhanced stability of factor VIII. Methods for making the recombinant factor VIII, pharmaceutical compositions containing the recombinant factor VIII, and use of the recombinant factor VIII for treating hemophilia A are also disclosed.
Owner:UNIVERSITY OF ROCHESTER
Recombinant factor viii having reduced inactivation by activated protein c
ActiveUS20090118185A1High catalytic efficiencyPromote localizationFactor VIIFungiProtein activationFactor ii
The present invention relates to a recombinant factor VIII that is characterized by one or more mutations within a region surrounding an activated protein C cleavage site, which one or more mutations result in a reduced rate of inactivation by activated protein C. Isolated nucleic acid molecules, recombinant expression vectors, and host cells suitable for expression of the recombinant factor VIII are also disclosed. The recombinant factor VIII can be used for the treatment of clotting disorders, such as hemophilia A.
Owner:UNIVERSITY OF ROCHESTER
Methods and Compositions for Treating Bleeding Disorders
InactiveUS20160060324A1Induce immune toleranceFactor VIIPeptide/protein ingredientsImmune toleranceB cell
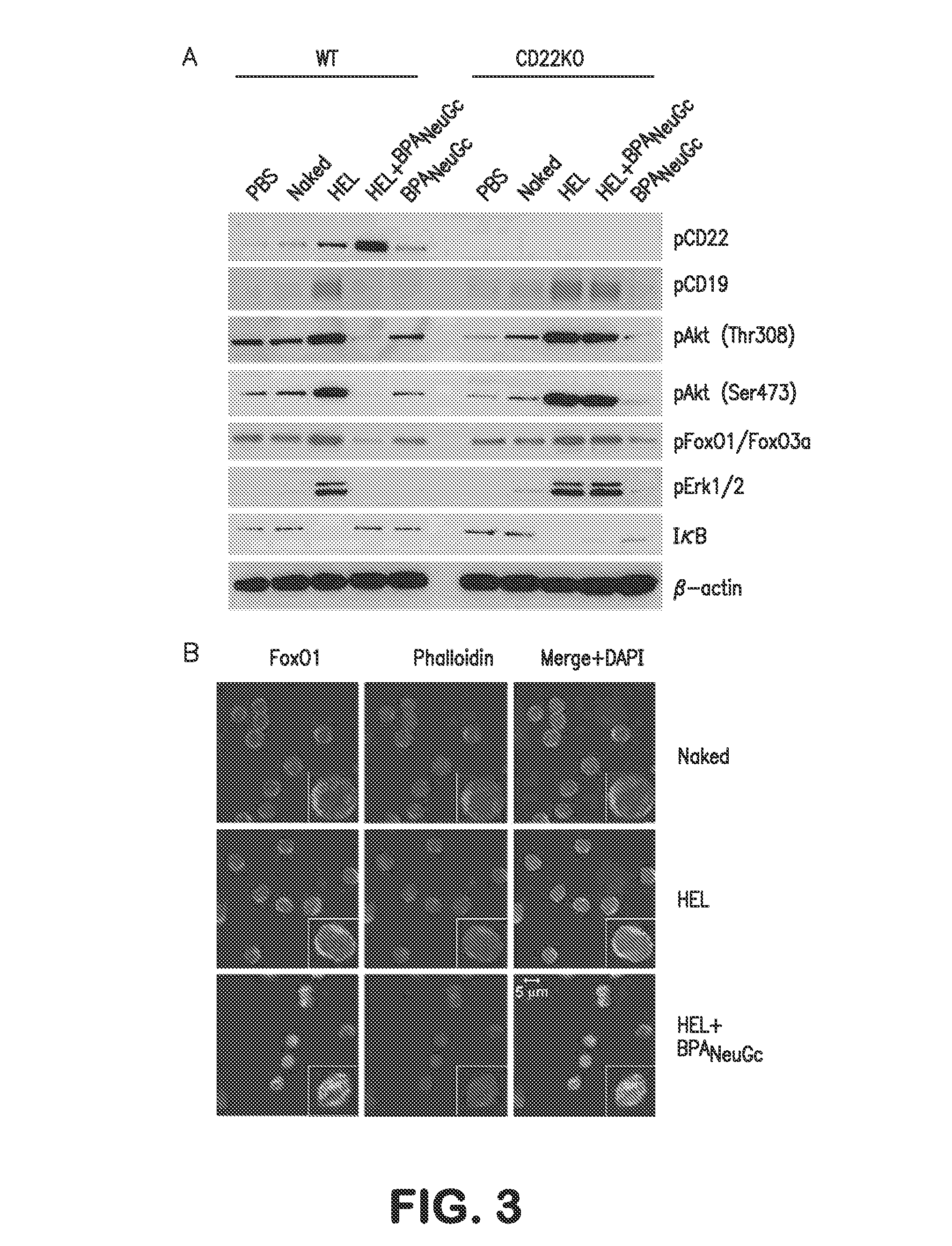
The present invention provides immune conjugates for inducing antigen specific immune tolerance to coagulation Factor VIII. The immune conjugates contain a FVIII protein or antigenic fragment that is conjugated to a binding moiety for a sialic acid binding Ig-like lectin (Siglec) expressed on B cells. The invention also provides methods of using the FVIII immune conjugates to induce immune tolerance to FVIII in a subject. Additionally provided in the invention are methods for treating bleeding disorders such as hemophilia A via the use of the FVIII immune conjugates and an unconjugated FVIII with coagulating activity.
Owner:THE SCRIPPS RES INST
Anti-idiotypic antibodies neutralizing the inhibitory activity of an inhibitory antibody directed against the c1 domain of factor viii
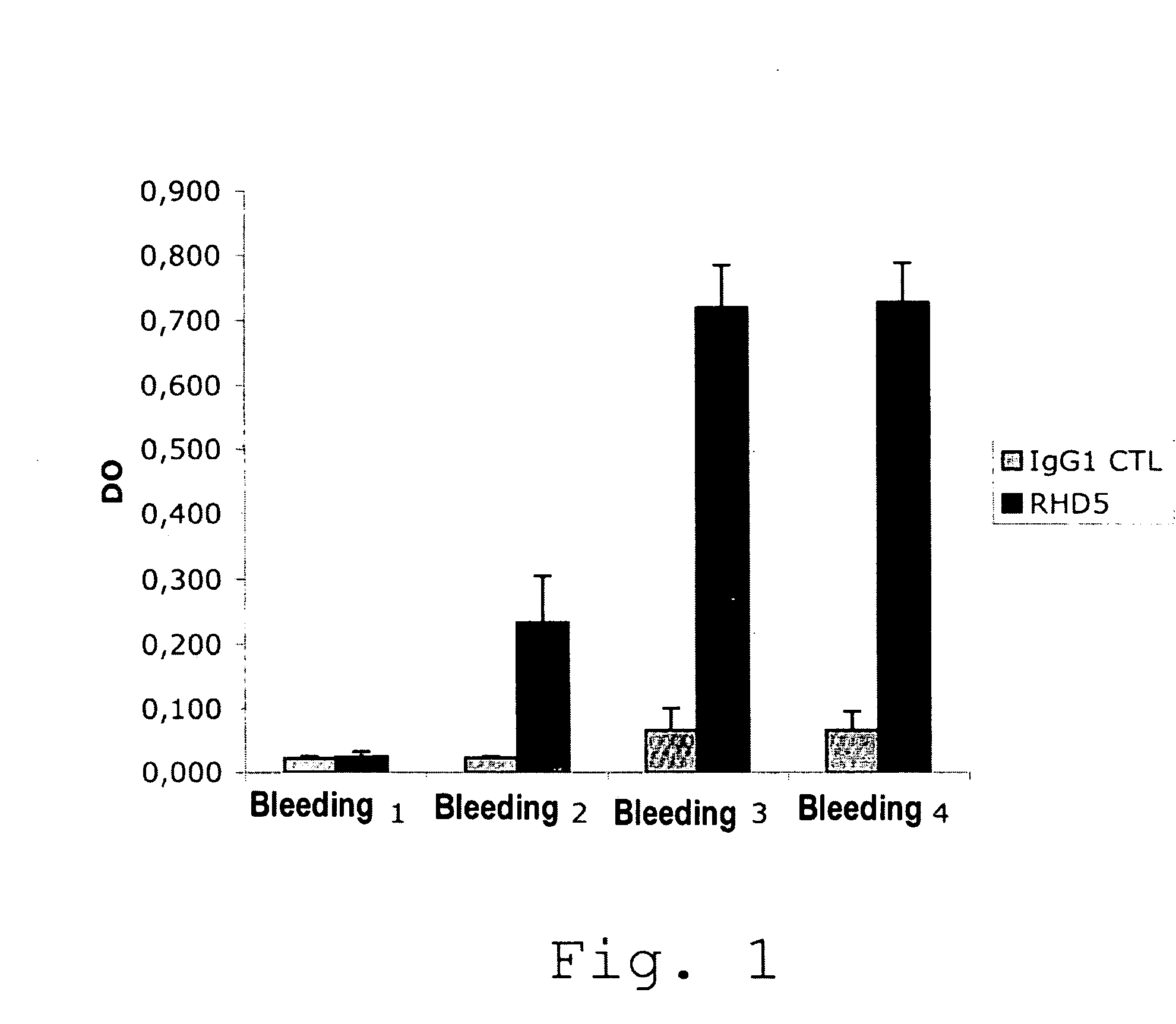
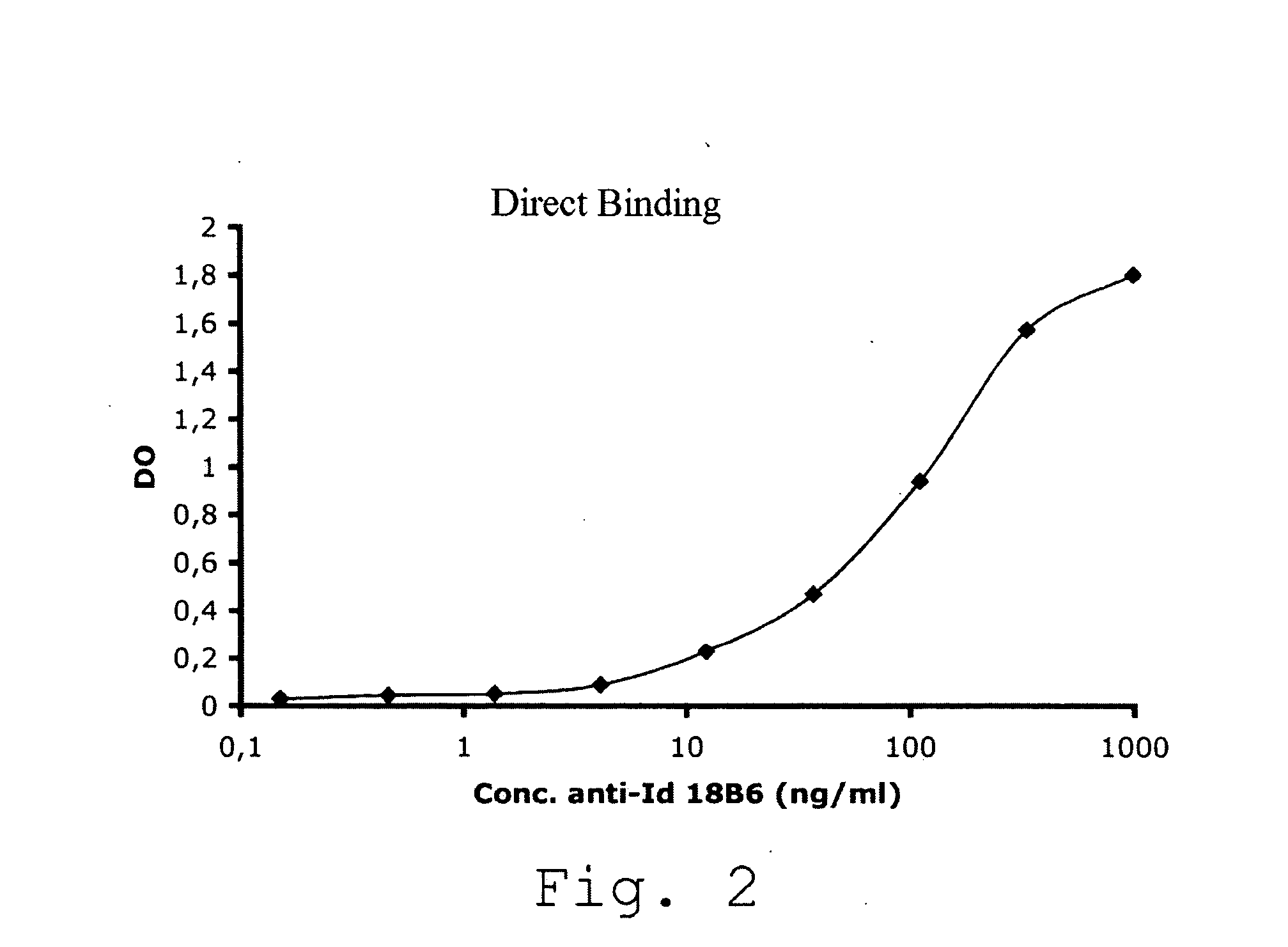
The present invention is related to a monoclonal anti-idiotypic antibody directed against a Factor VIII inhibitor antibody binding to the C1 domain of Factor VIII, as well as to a cell line producing this monoclonal anti-idiotypic antibody, to the use of this monoclonal anti-idiotypic antibody as medicament, and more particularly to the use thereof for manufacturing a medicament intended for the treatment of haemophilia A.
Owner:LFB BIOTECH
Suppression of immune response to factor viii in hemophilia a patients
Owner:PUGET SOUND BLOOD CENT
Targeting Tissue Factor To Activated Platelets
ActiveUS20160272710A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsTissue factorPlatelet
The current invention relates to procoagulant fusion proteins, polynucleotides that encode said fusion proteins and cells that expresses said fusion proteins. Furthermore, the current invention relates to fusion proteins for use as a medicament. Individuals that have a coagulopathy, such as haemophilia A and B with or without inhibitors, may be treated with fusions proteins of the current invention.
Owner:NOVO NORDISK AS
Anti-idiotypic antibody neutralizing the inhibitor activity of a factor viii inhibitor antibody
InactiveUS20070065425A1Reduce antibody immunogenicityImprove efficiencyAnimal cellsSugar derivativesFactor VIII inhibitorAnti-idiotypic antibodies
The present invention is related to a monoclonal anti-idiotypic antibody directed against a Factor VIII inhibitor antibody binding to the domain A2 of Factor VIII, and to a cell line producing this monoclonal anti-idiotypic antibody, to the use of this monoclonal anti-idiotypic antibody as drug, and more particularly, to its use for the manufacturing of a drug to be used for the treatment of haemophilia A.
Owner:LFB BIOTECH
Severe hemophilia A disease-causing gene mutation detection kit
ActiveCN102943114AImprove stabilityImprove efficiencyMicrobiological testing/measurementInteinDNA Polymerase Inhibitor
The invention belongs to the field of biotechnology and biomedicine, and particularly relates to a kit for detecting inversion mutation of intron 22 and intron 1 of a severe hemophilia A disease-causing gene. The severe hemophilia A disease-causing gene mutation detection kit comprises Inv22 PCR (polymerase chain reaction) liquid and Inv1 PCR liquid, the Inv22 PCR liquid comprises three primers for detecting gene inversion of the intron 22 of the severe hemophilia A disease-causing gene, and the Inv1 PCR liquid comprises three primers for detecting gene inversion of the intron 1 of the severe hemophilia A disease-causing gene. Long fragment amplification stability of Inv22, detection sensitivity, PCR product quantity and electrophoresis detection abundance are improved, the problems of short preservation time, poor temperature tolerance and the like of reagents are solved by further adding Taq DNA (deoxyribonucleic acid) polymerase inhibitors, and stability of clinical application of the kit is improved.
Owner:亚能生物技术(深圳)有限公司
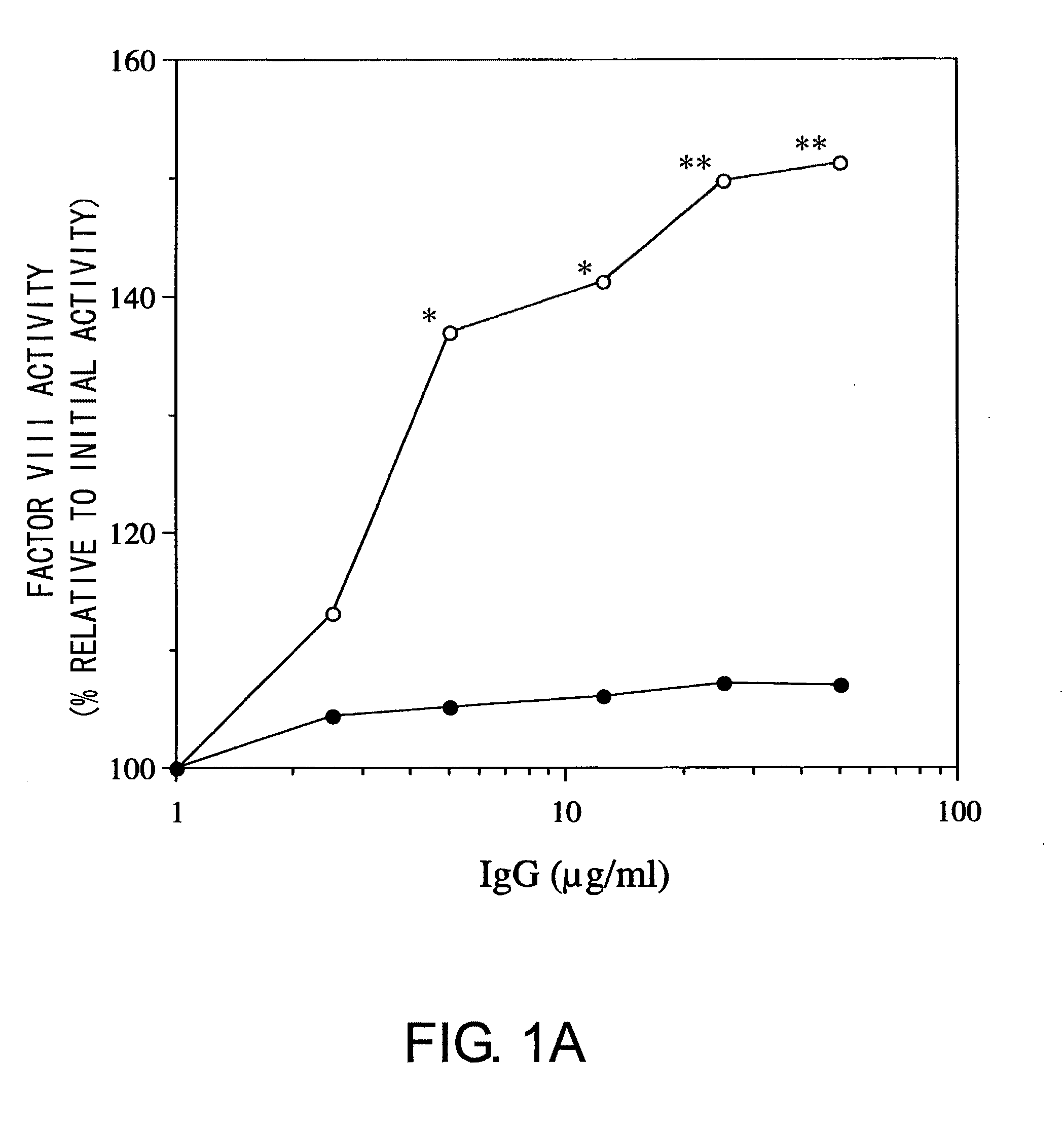
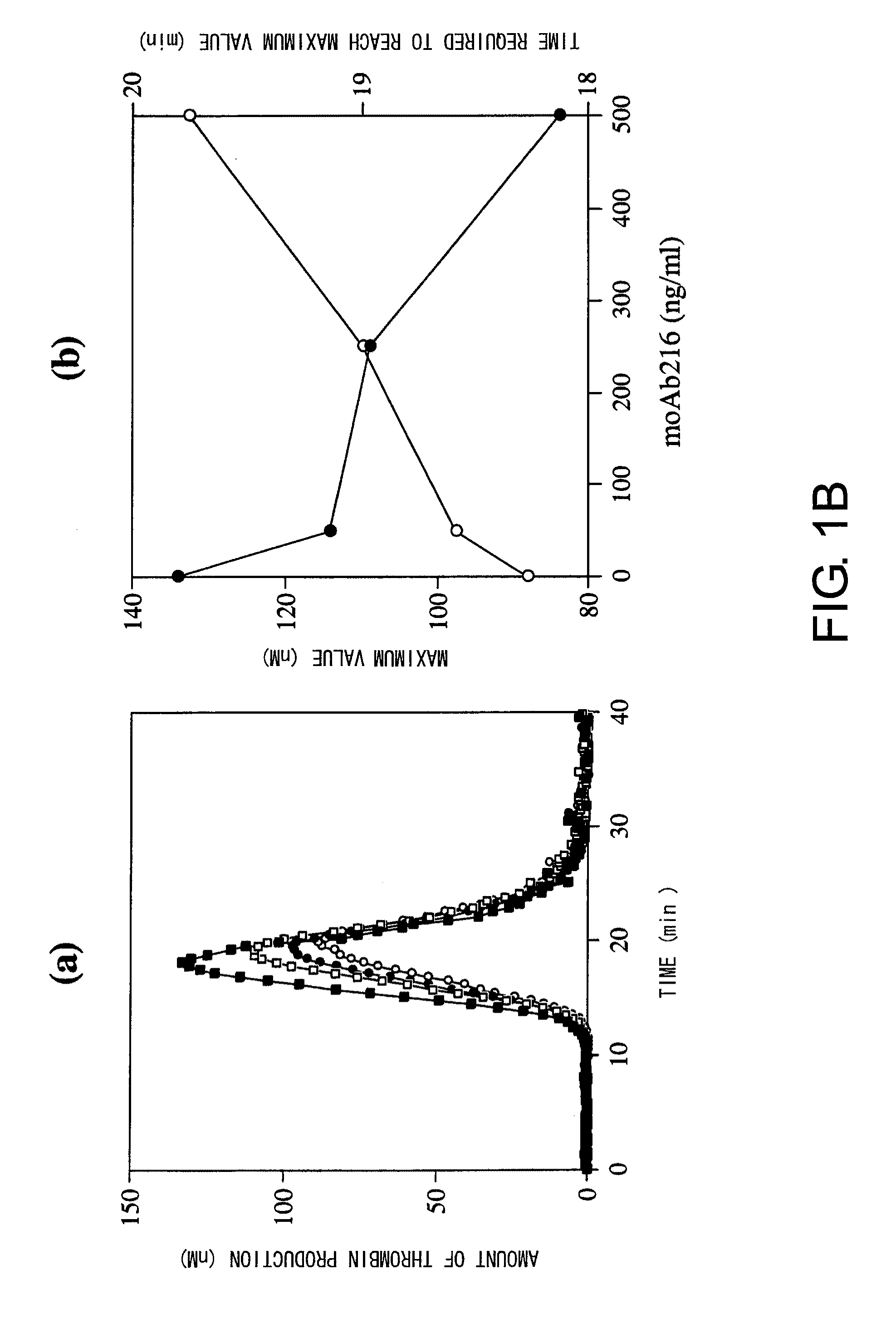
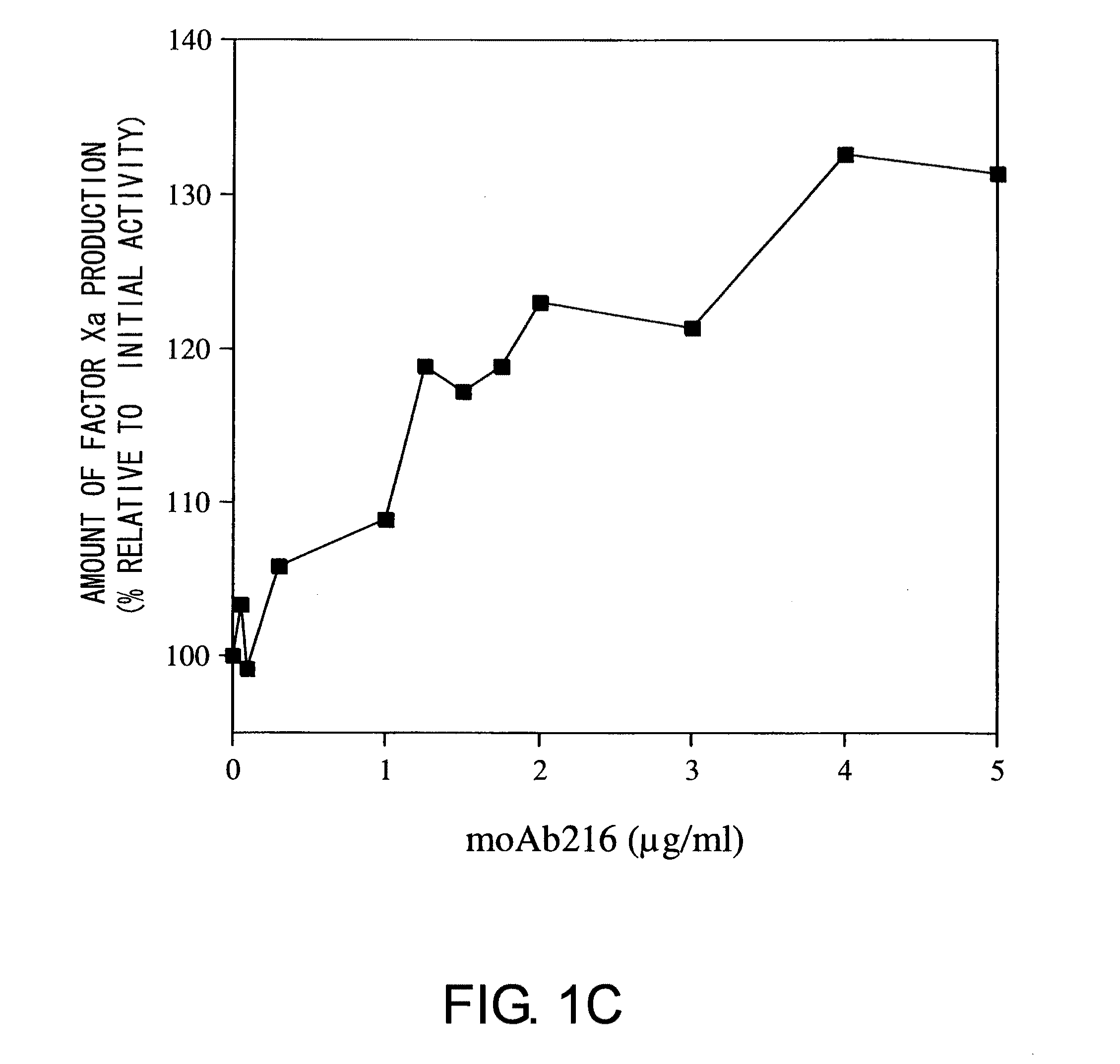
Blood Coagulation Factor VIII Activation-Enhancing Antibodies
ActiveUS20090297503A1Increases coagulation-enhancing activity of Factor VIIIHigh activityImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIVon willebrand
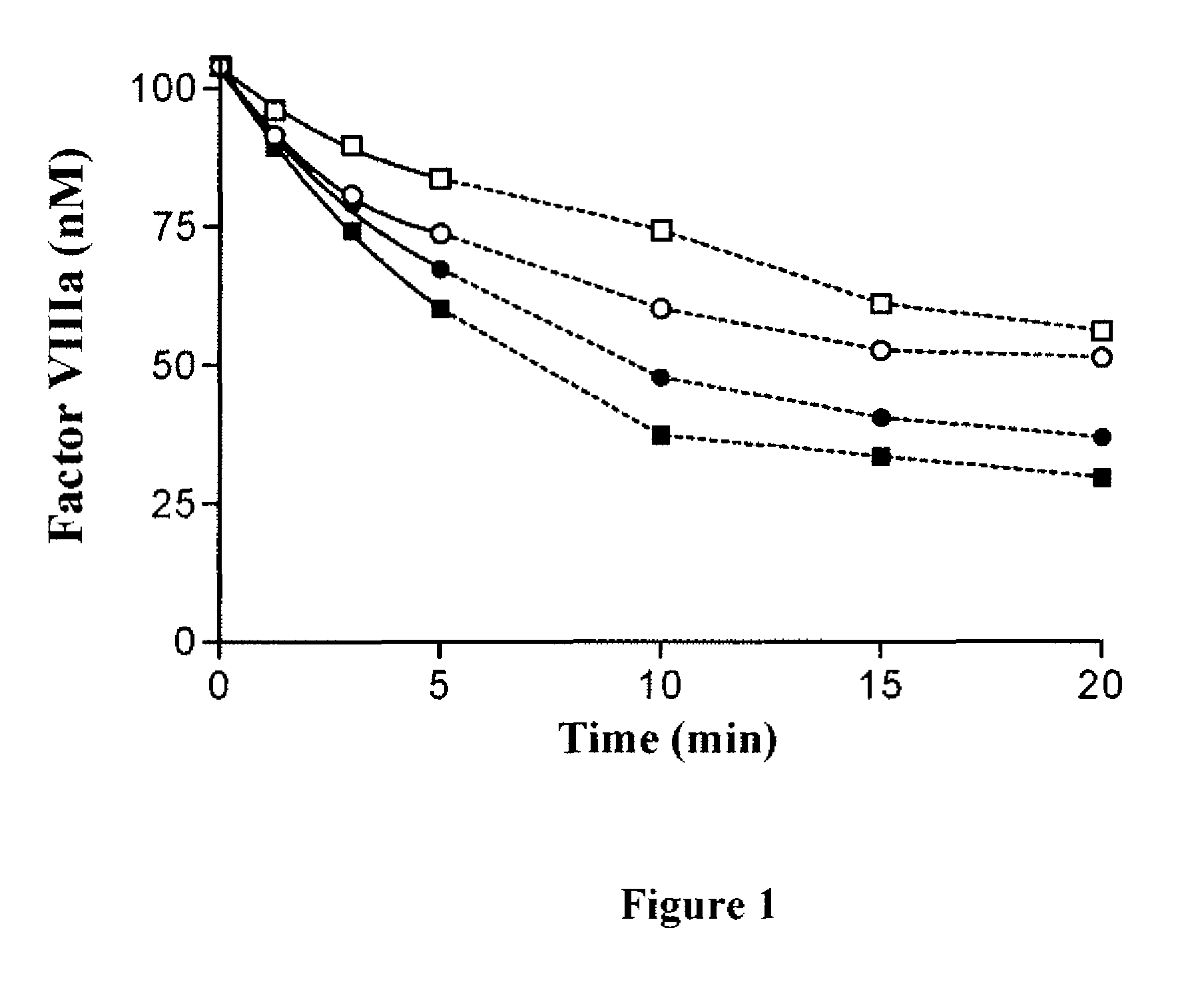
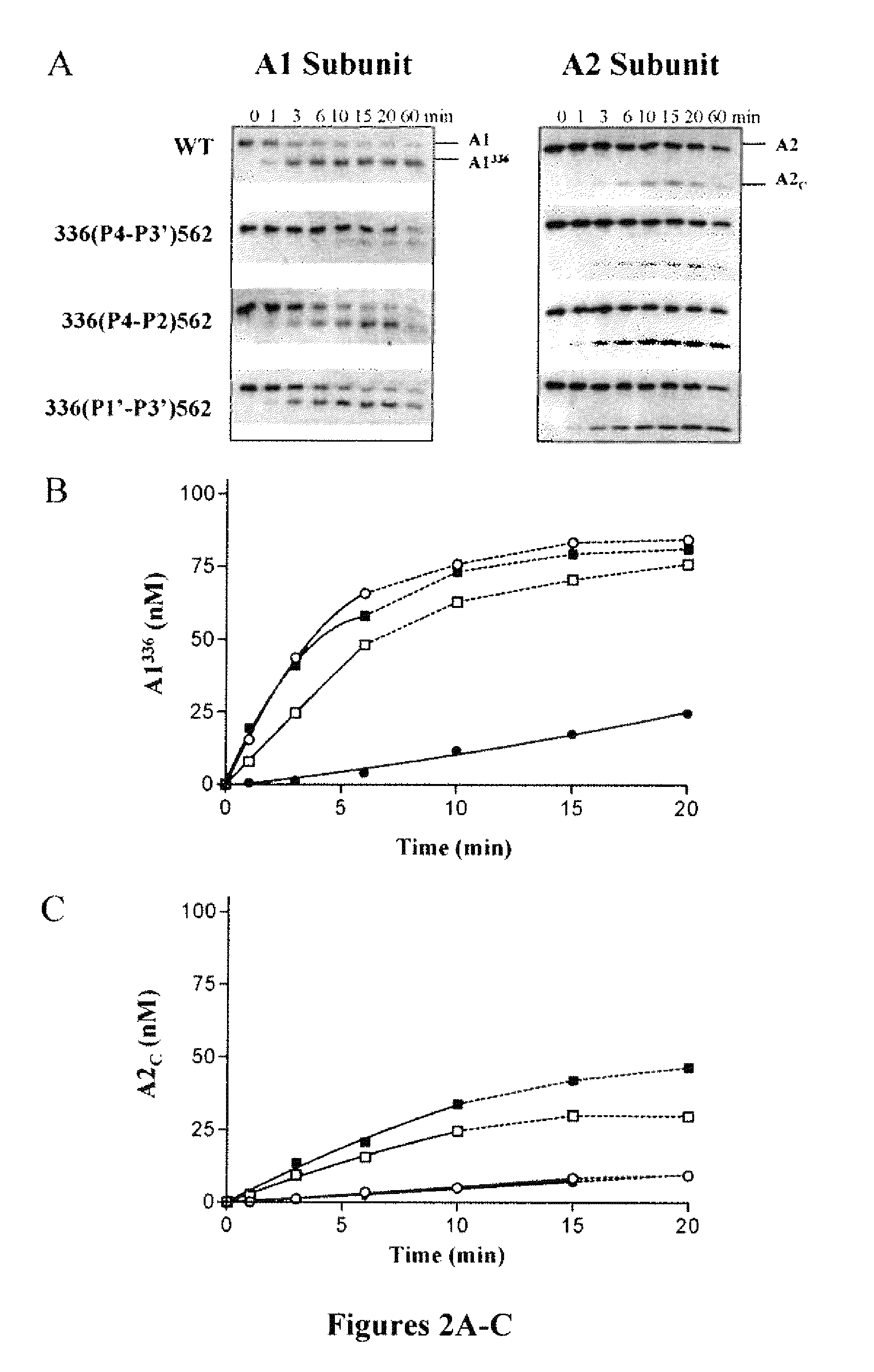
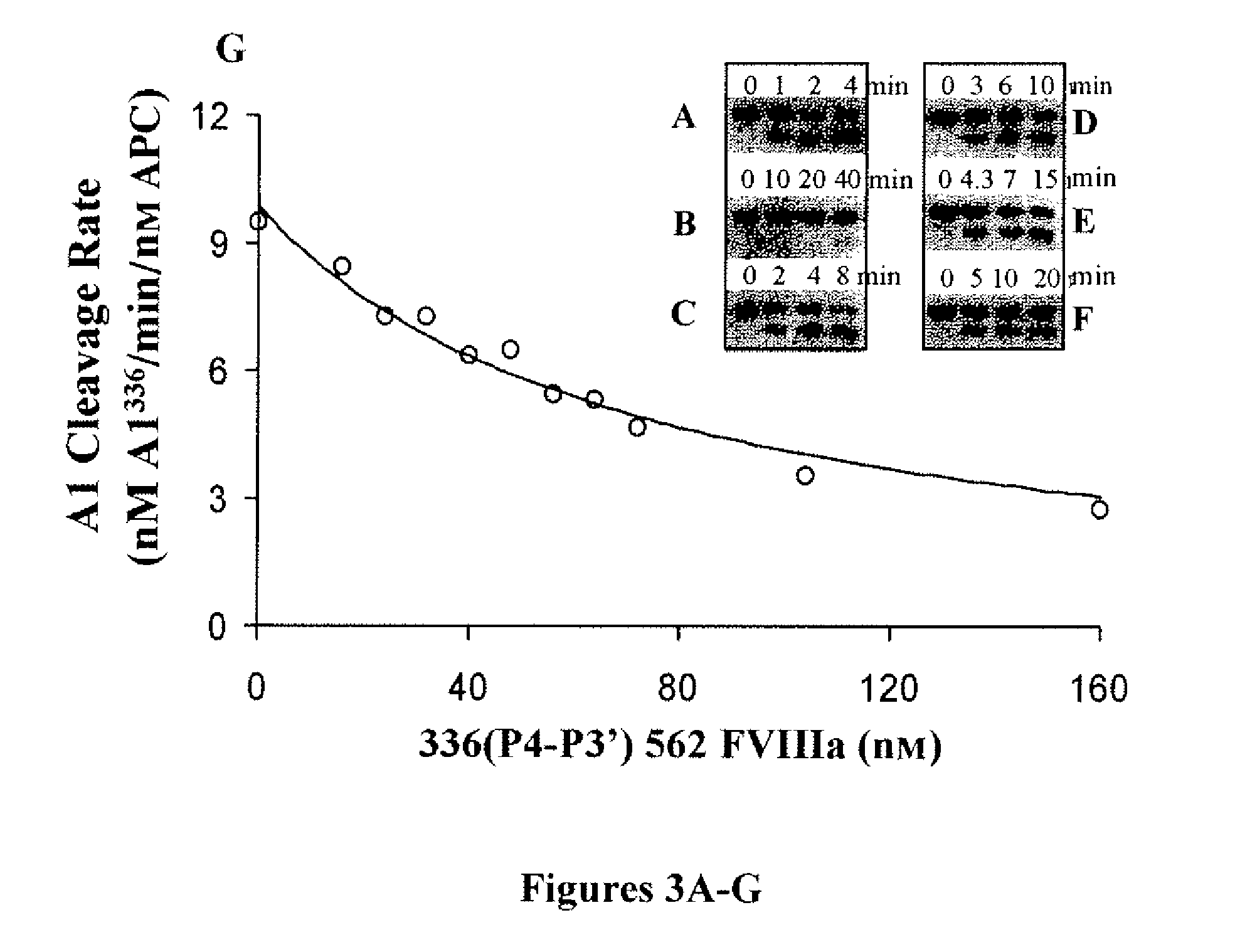
For the first time, the present invention provides antibodies that enhance the generation of activated blood coagulation factor VIII. The antibodies enhance the cleavage of blood coagulation factor VIII at the Arg of position 372 and suppress the cleavage at the Arg of position 336 by recognizing and binding to the A2 domain of blood coagulation Factor VIII. Such antibodies are expected to be useful in preventing or treating diseases that develop or progress due to decrease or loss of the blood coagulation factor VIII activity, for example, hemophilia A, acquired hemophilia, and von Willebrand's disease.
Owner:CHUGAI PHARMA CO LTD +1
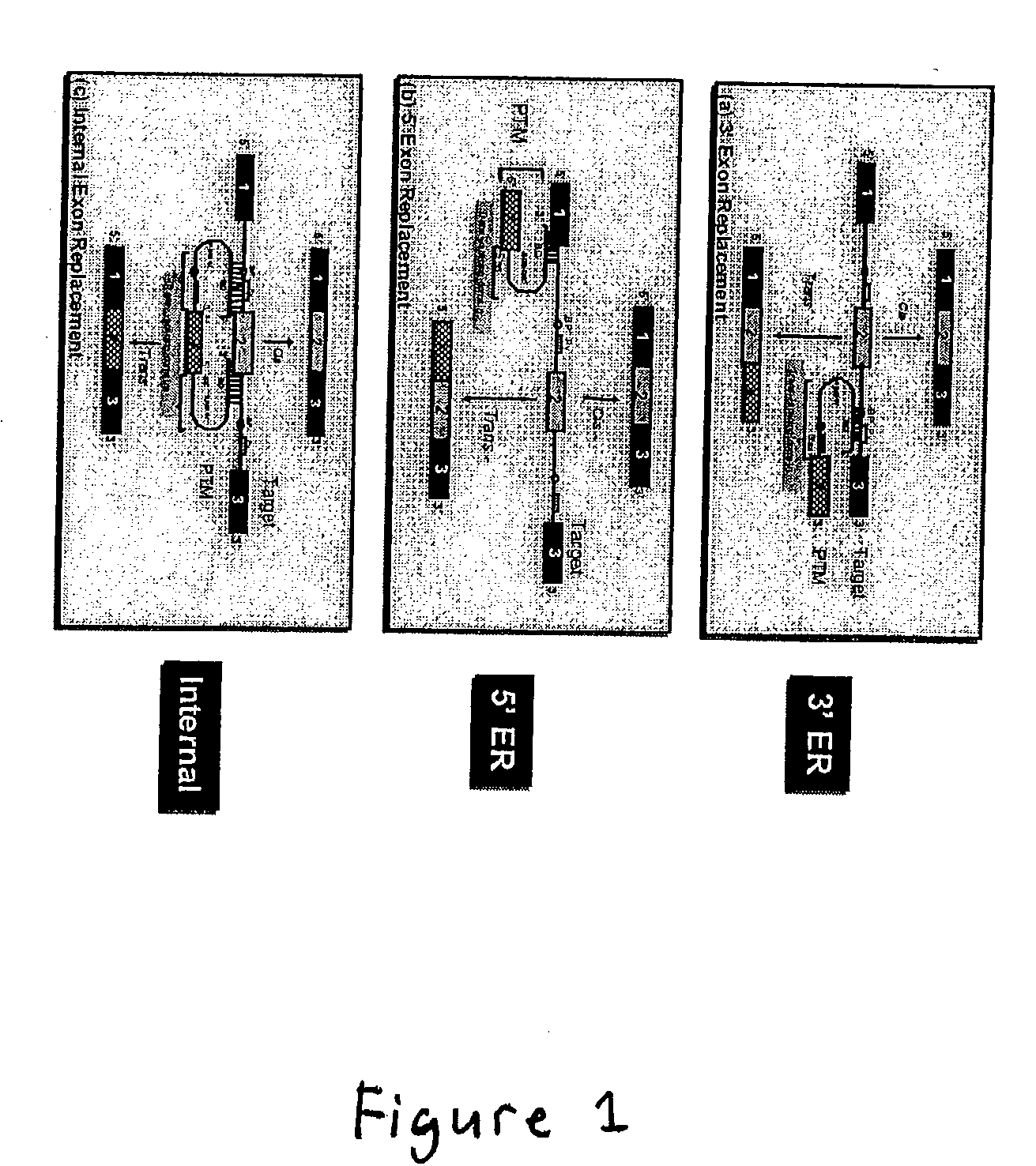
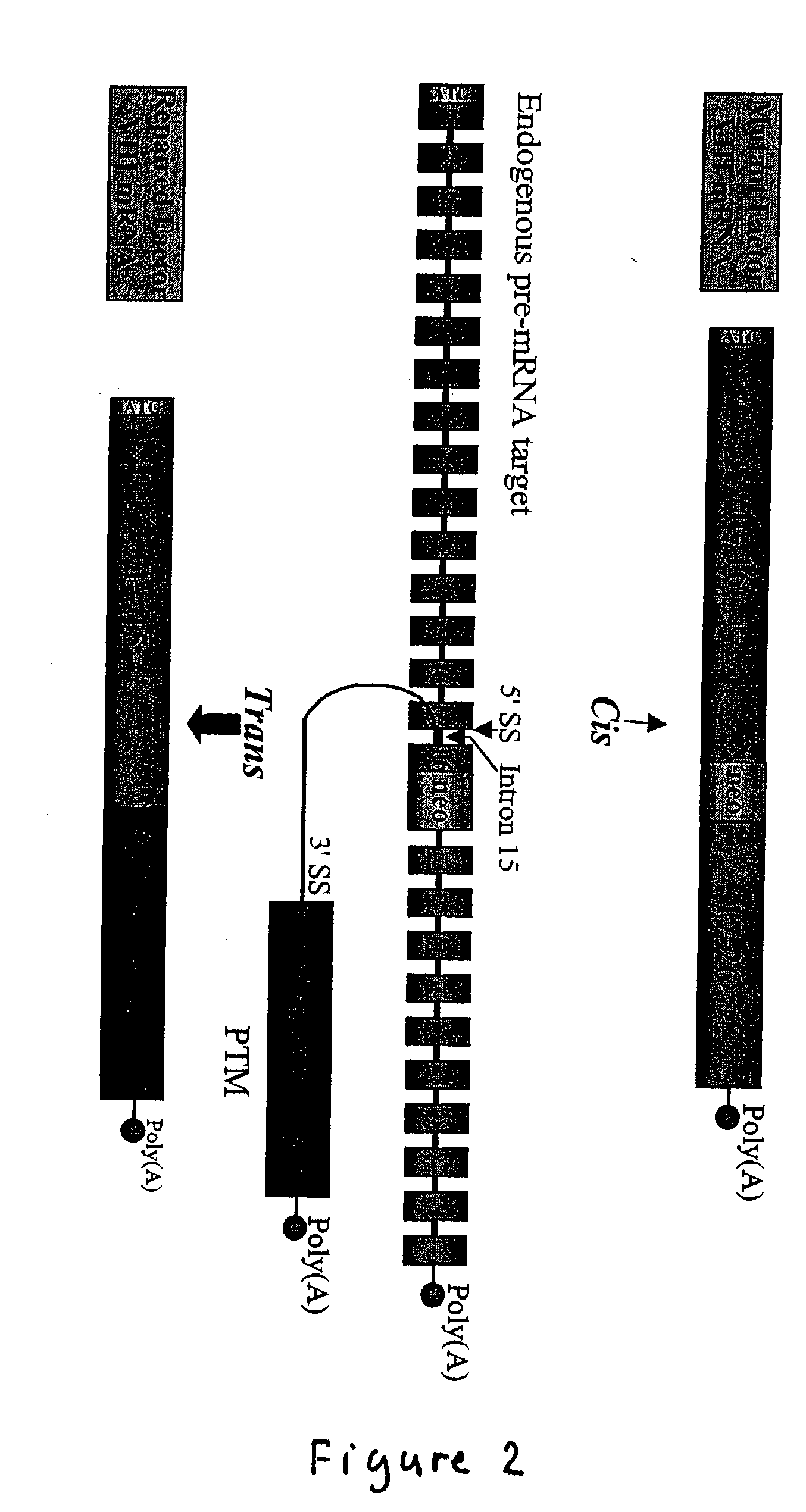
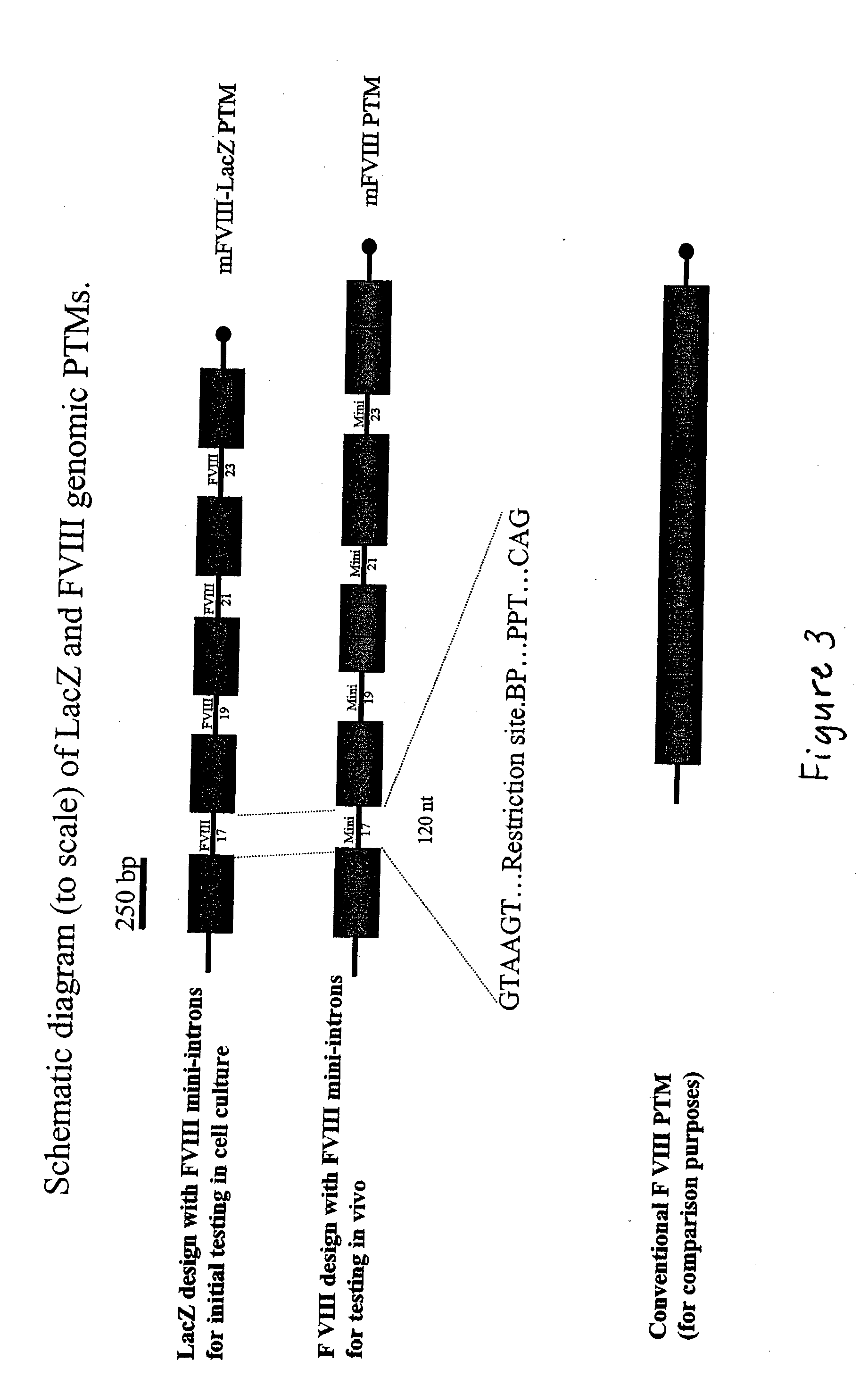
Correction of factor VIII genetic defects using spliceosome mediated RNA trans splicing
InactiveUS20040126774A1Splicing alterationMicrobiological testing/measurementRNA Trans-SplicingVector system
The present invention provides methods and compositions for generating novel nucleic acid molecules through targeted spliceosomal mediated trans-splicing. The compositions of the invention include pre-trans-splicing molecules (PTMs) designed to interact with a target precursor messenger RNA molecule (target pre-mRNA) and mediate a trans-splicing reaction resulting in the generation of a novel chimeric RNA molecule (chimeric RNA). In particular, the PTMs of the present invention are genetically engineered to interact with factor VIII (FVIII) target pre-mRNA so as to result in correction of clotting FVIII genetic defects responsible for hemophilia A. The compositions of the invention further include recombinant vector systems capable of expressing the PTMs of the invention and cells expressing said PTMs. The methods of the invention encompass contacting the PTMs of the invention with a FVIII target pre-mRNA under conditions in which a portion of the PTM is trans-spliced to a portion of the target pre-mRNA to form a RNA molecule wherein the genetic defect in the FVIII gene has been corrected. The methods and compositions of the present invention can be used in gene therapy for correction of FVIII disorders such as hemophilia A.
Owner:VIRXSYS
Factor IX variants with clotting activity in absence of their cofactor and/or with increased F.IX clotting activity and their use for treating bleeding disorders
The present invention relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has increased F.IX clotting activity compared to wildtype. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Blood coagulation factor VIII activation-enhancing antibodies
ActiveUS8252287B2Increases coagulation-enhancing activity of Factor VIIIHigh activityImmunoglobulins against blood coagulation factorsFactor VIIBlood coagulation factor VIIIVon willebrand
For the first time, the present invention provides antibodies that enhance the generation of activated blood coagulation factor VIII. The antibodies enhance the cleavage of blood coagulation factor VIII at the Arg of position 372 and suppress the cleavage at the Arg of position 336 by recognizing and binding to the A2 domain of blood coagulation Factor VIII. Such antibodies are expected to be useful in preventing or treating diseases that develop or progress due to decrease or loss of the blood coagulation factor VIII activity, for example, hemophilia A, acquired hemophilia, and von Willebrand's disease.
Owner:CHUGAI PHARMA CO LTD +1
Antibody capable of neutralizing substance having activity alternative to function of coagulation factor VIII (FVIII)
It is tried to produce an antibody capable of neutralizing the activity of a substance having an activity alternative to the function of FVIII, for the purpose of using the antibody in a method for measuring the reactivity of FVIII in the presence of the substance having the activity alternative to the function of FVIII. As the result, it is found that, when the produced antibody is used, the activity of FVIII in plasma from a hemophilia A patient can be evaluated accurately in an APTT-based one-stage clotting assay. It is also found that, when the produced antibody is used, the titer of an FVIII inhibitor in plasma from an FVIII inhibitor-carrying hemophilia A patient can be evaluated accurately in an APTT-based Bethesda assay.
Owner:CHUGAI PHARMA CO LTD
Novel VIII Factors for the Treatment of Type A Hemophilia
InactiveUS20100311659A1Improve the problemIncrease capacityFactor VIIPeptide/protein ingredientsCancer researchIn patient
Owner:BIOMETHODES R L +1
Anti-idiotypic antibody neutralizing the inhibitor activity of a factor VIII inhibitor antibody
InactiveUS8071094B2Reduce antibody immunogenicityImprove efficiencySugar derivativesAntibody ingredientsFactor VIII inhibitorAnti-idiotypic antibodies
The present invention is related to a monoclonal anti-idiotypic antibody directed against a Factor VIII inhibitor antibody binding to the domain A2 of Factor VIII, and to a cell line producing this monoclonal anti-idiotypic antibody, to the use of this monoclonal anti-idiotypic antibody as drug, and more particularly, to its use for the manufacturing of a drug to be used for the treatment of haemophilia A.
Owner:LFB BIOTECH
Factor viii sequences
ActiveUS20170049859A1Shorten the timeImprove the level ofFactor VIIPeptide/protein ingredientsGene deliveryNucleotide
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +1
Therapeutic factor VIII antibodies
InactiveUS9062115B2Extended service lifeExcessively generatedImmunoglobulins against blood coagulation factorsPeptide/protein ingredientsHalf-lifeHaemophilia A
Owner:NOVO NORDISK AS
Factor IX variants with clotting activity in absence of their cofactor and their use for treating bleeding disorders
The present invention relates to variants of a vitamin K-dependent serine protease of the coagulation cascade, preferably variants of factor IX (F.IX), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Targeting tissue factor to activated platelets
InactiveUS20120178908A1Peptide/protein ingredientsMammal material medical ingredientsTissue factorPlatelet
The current invention relates to procoagulant fusion proteins, polynucleotides that encode said fusion proteins and cells that expresses said fusion proteins. Furthermore, the current invention relates to fusion proteins for use as a medicament. Individuals that have a coagulopathy, such as haemophilia A and B with or without inhibitors, may be treated with fusions proteins of the current invention.
Owner:NOVO NORDISK AS
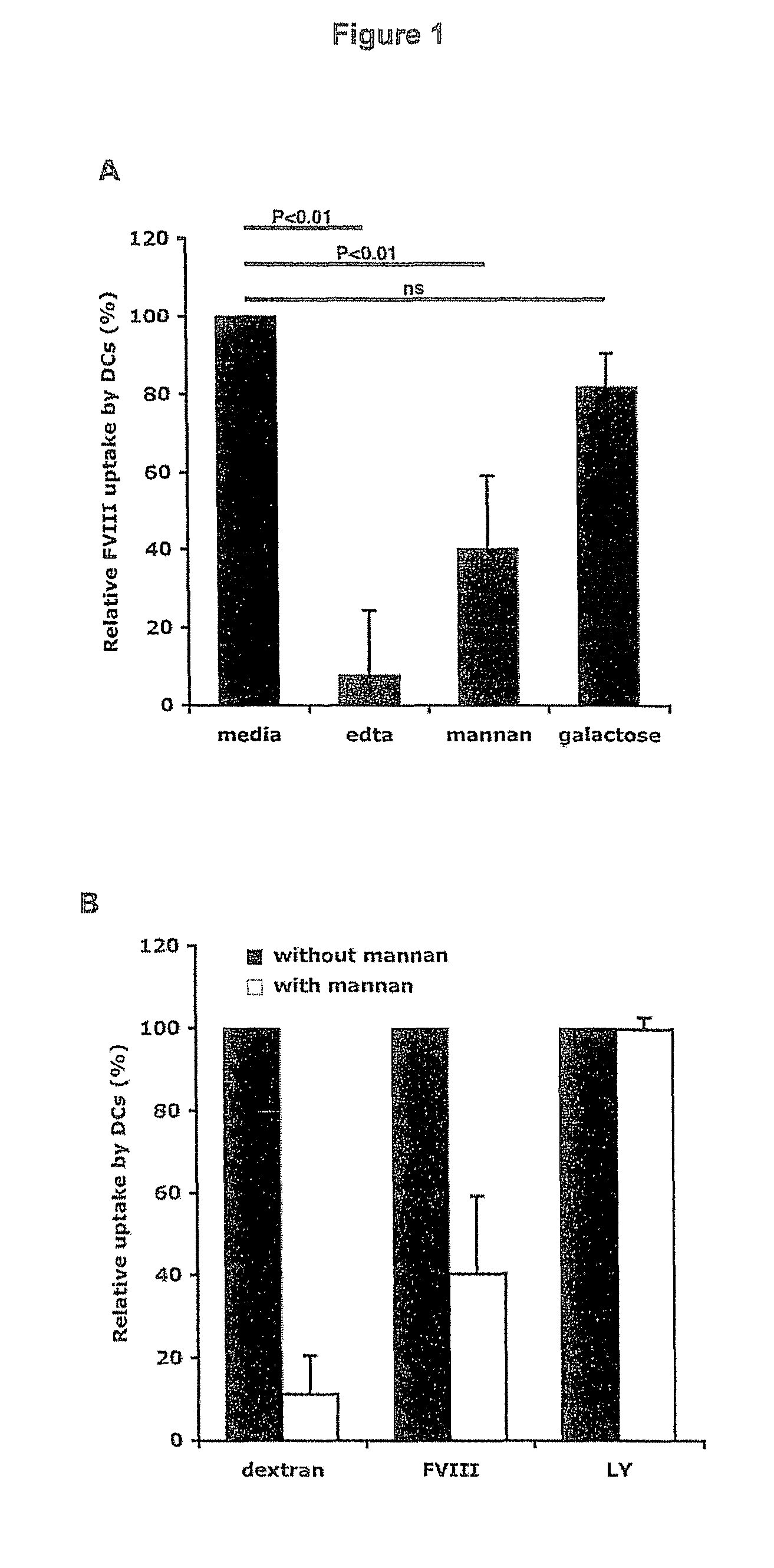
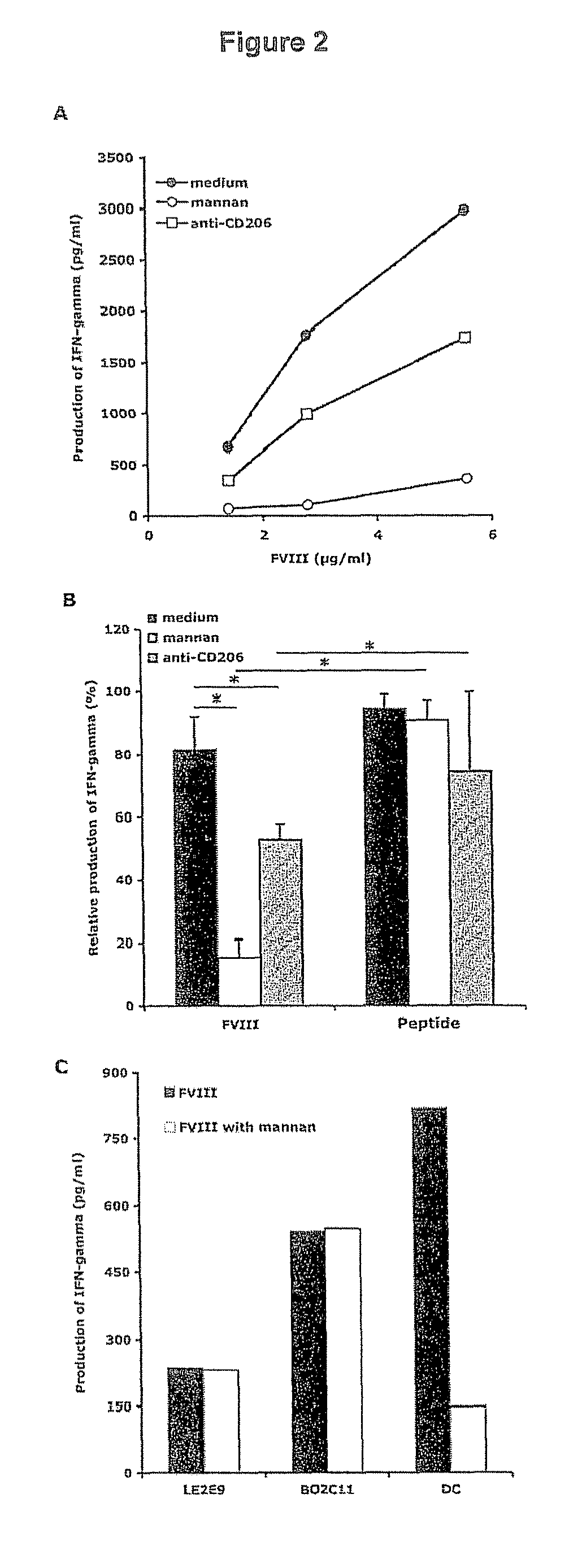
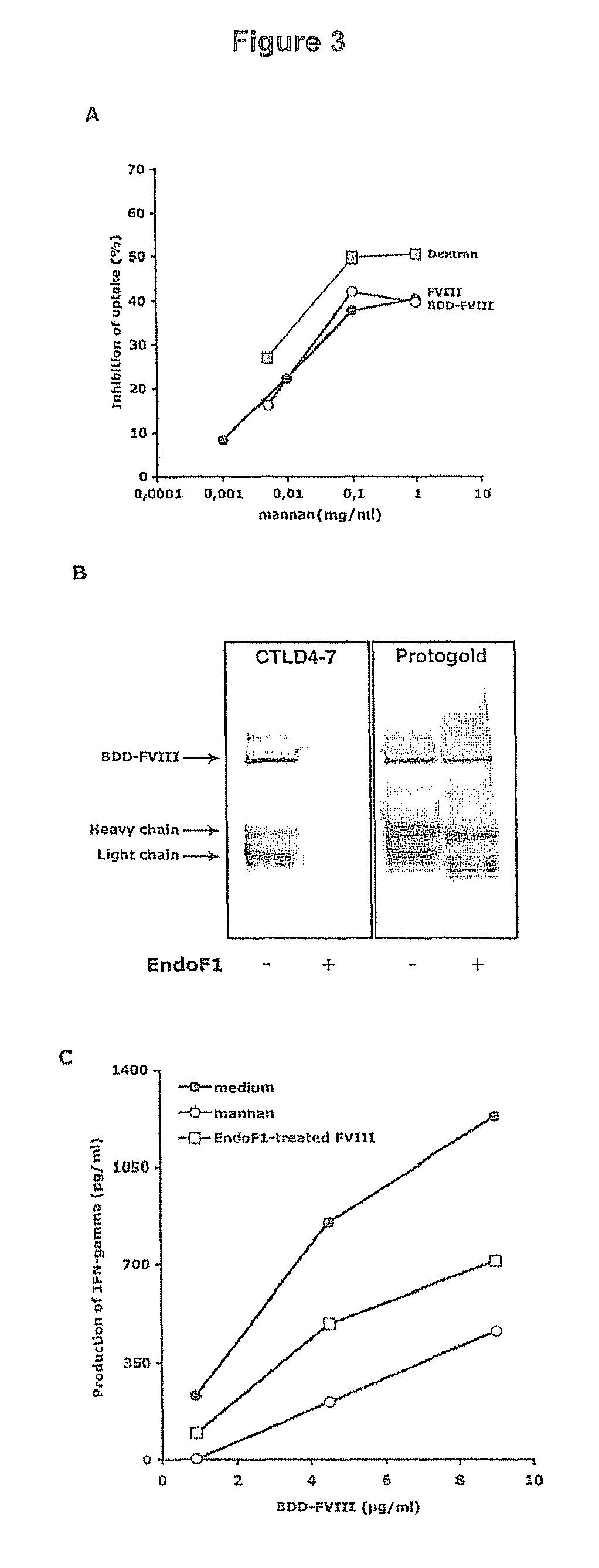
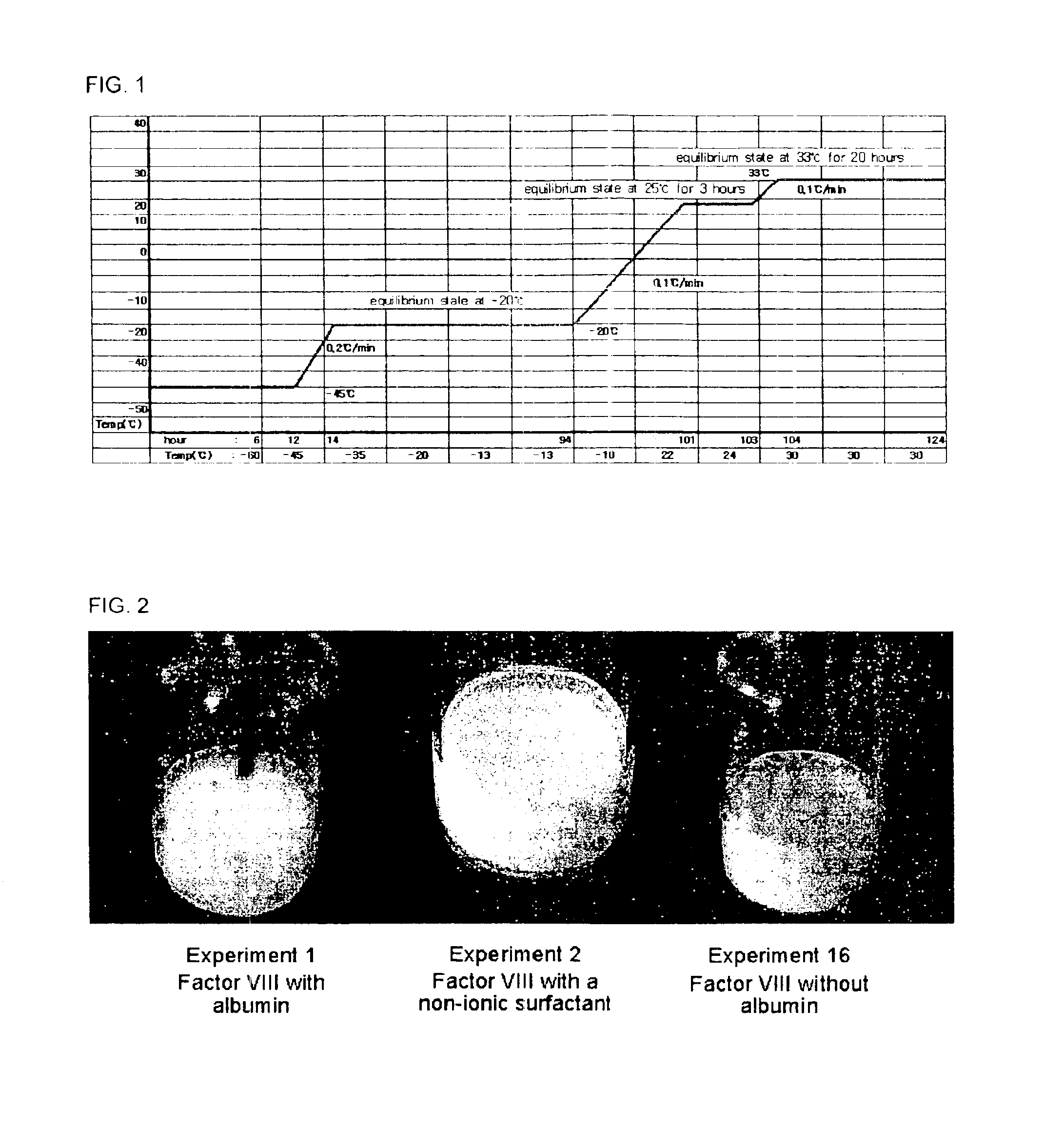
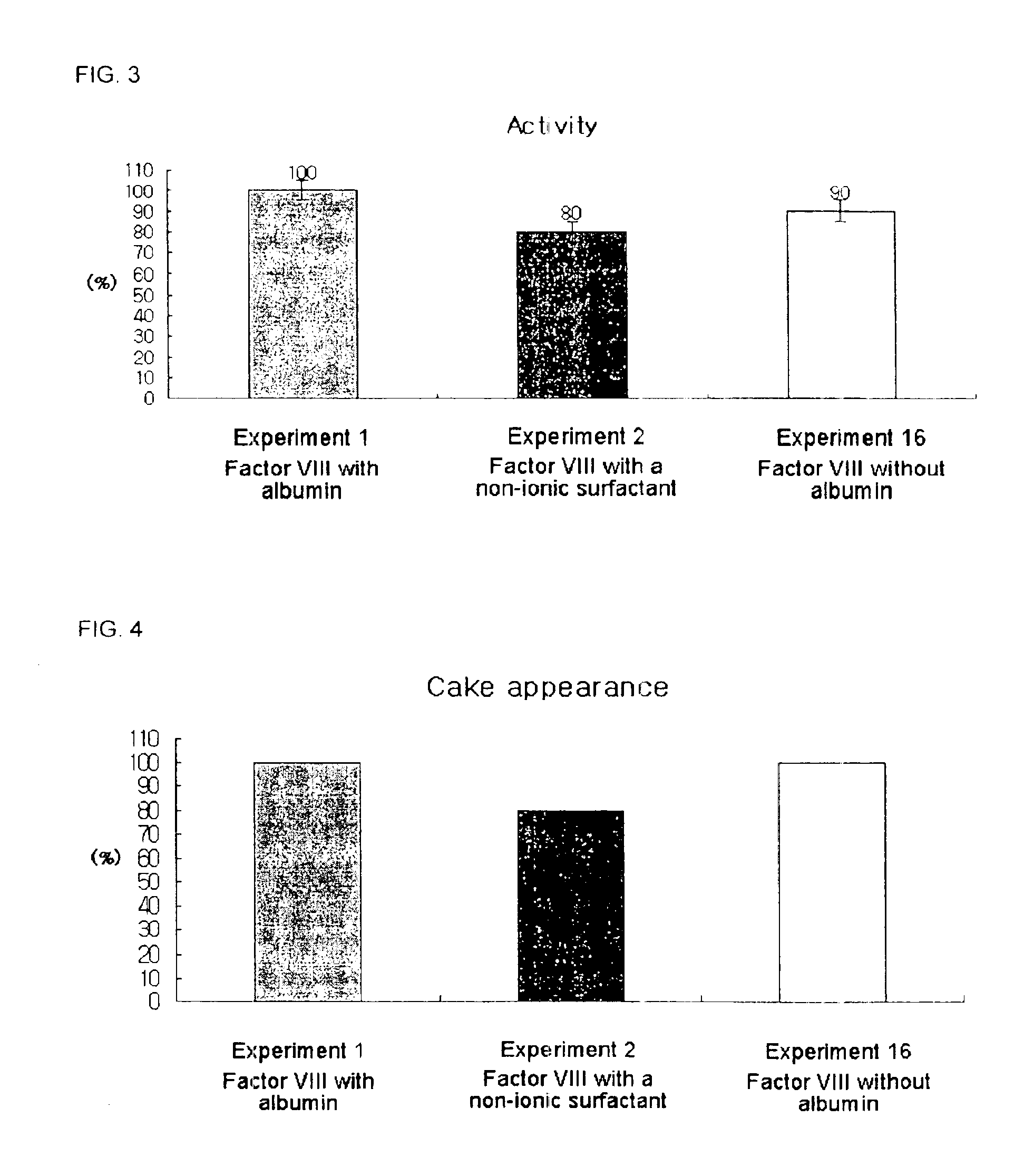
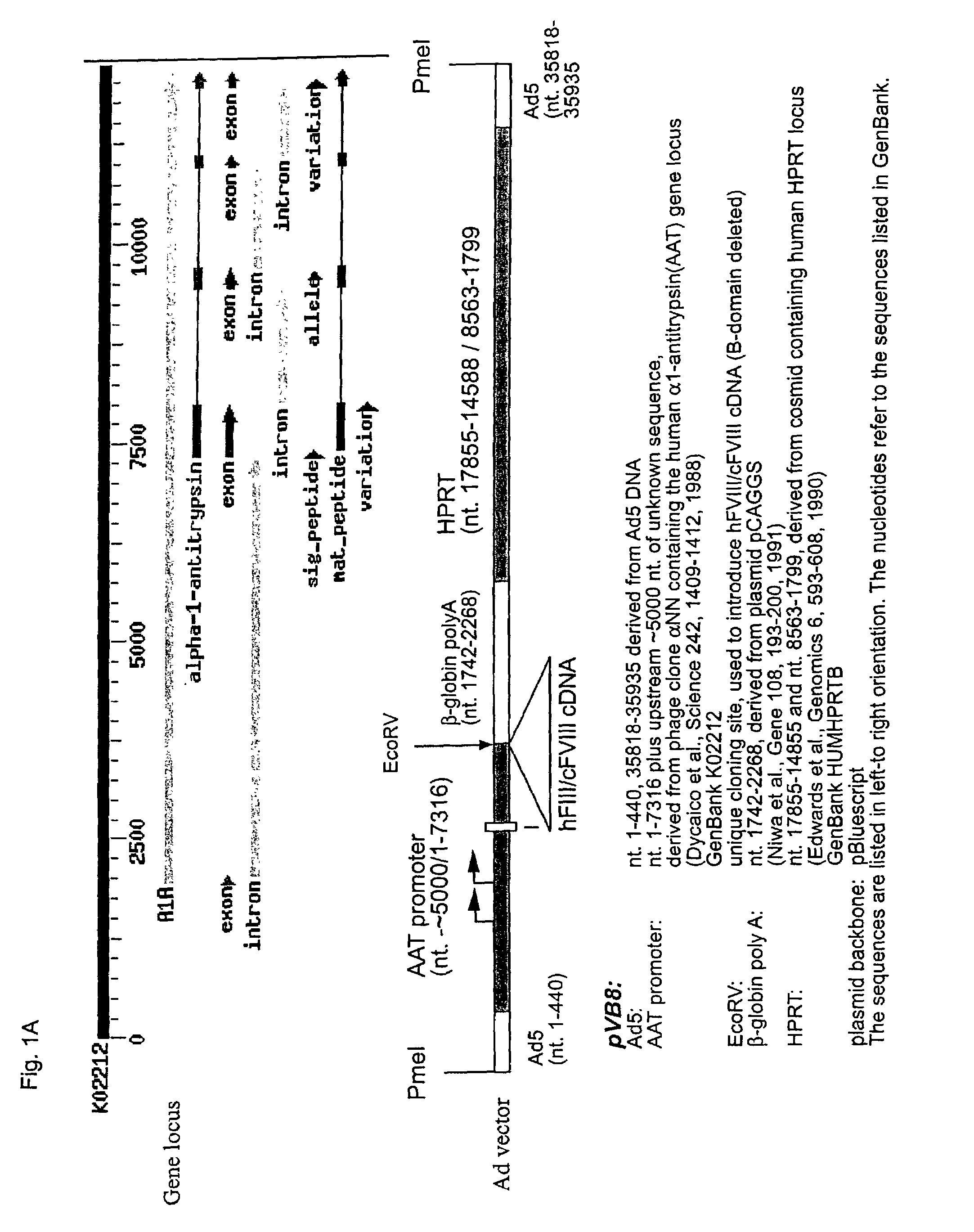
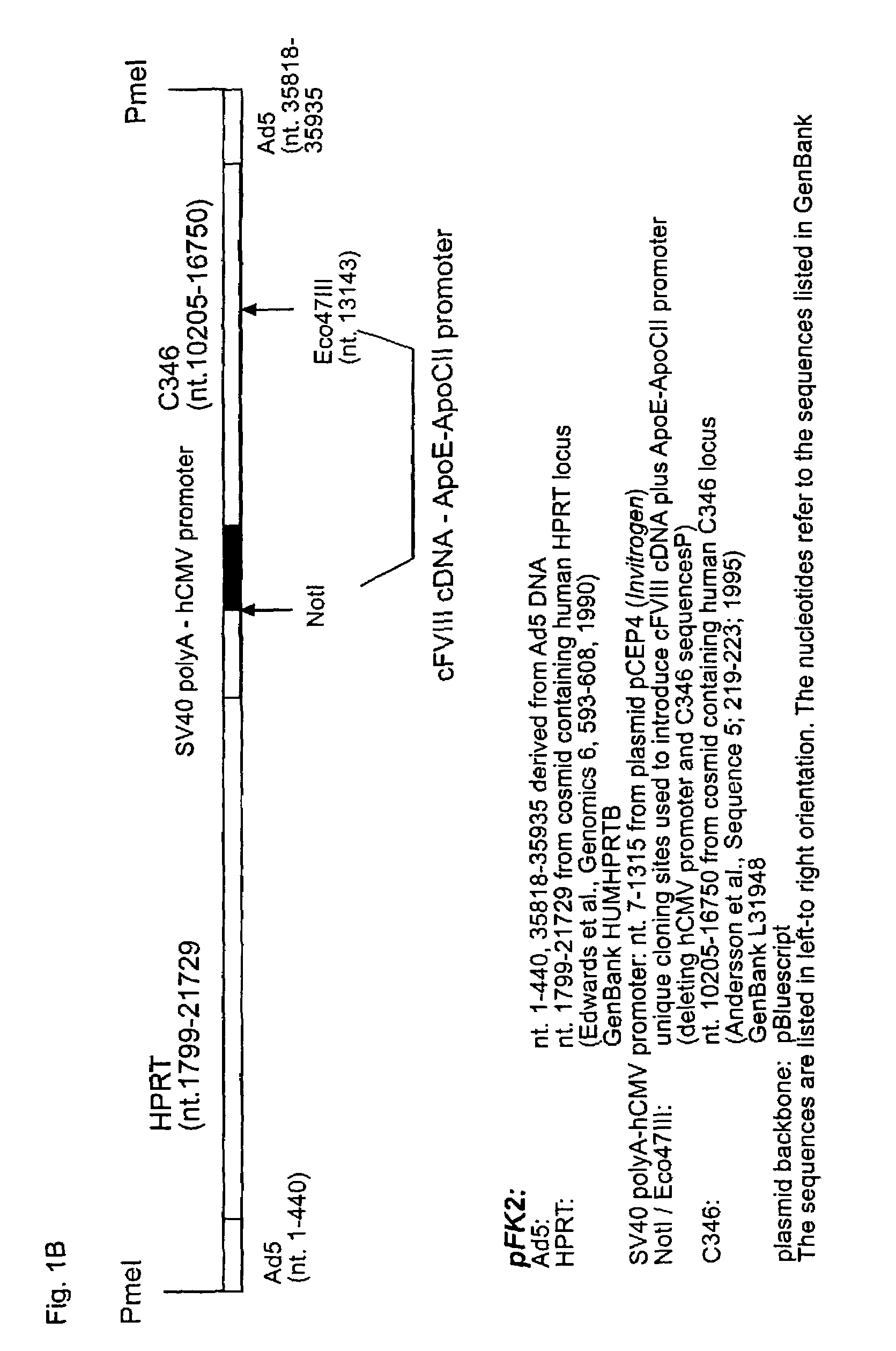
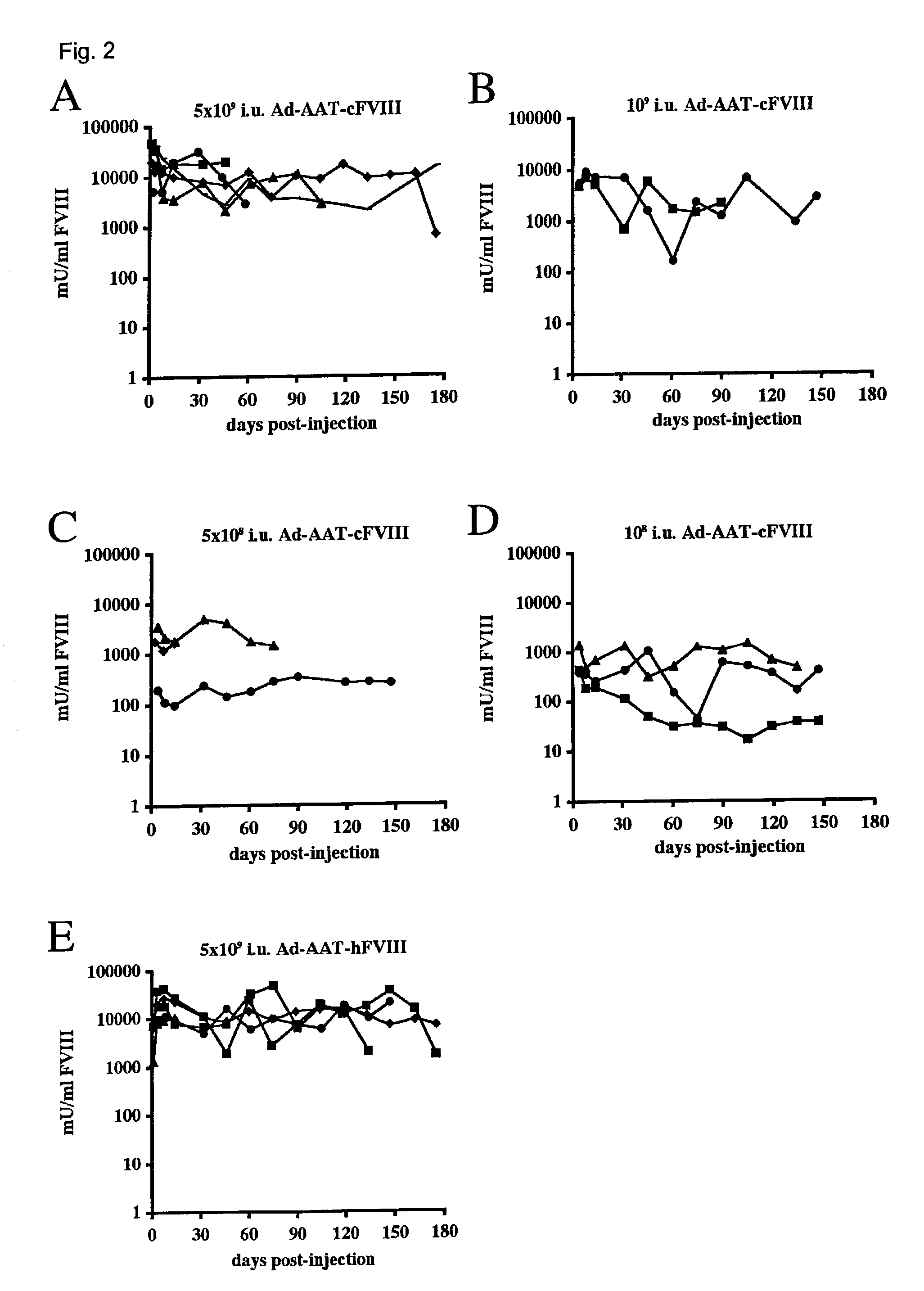
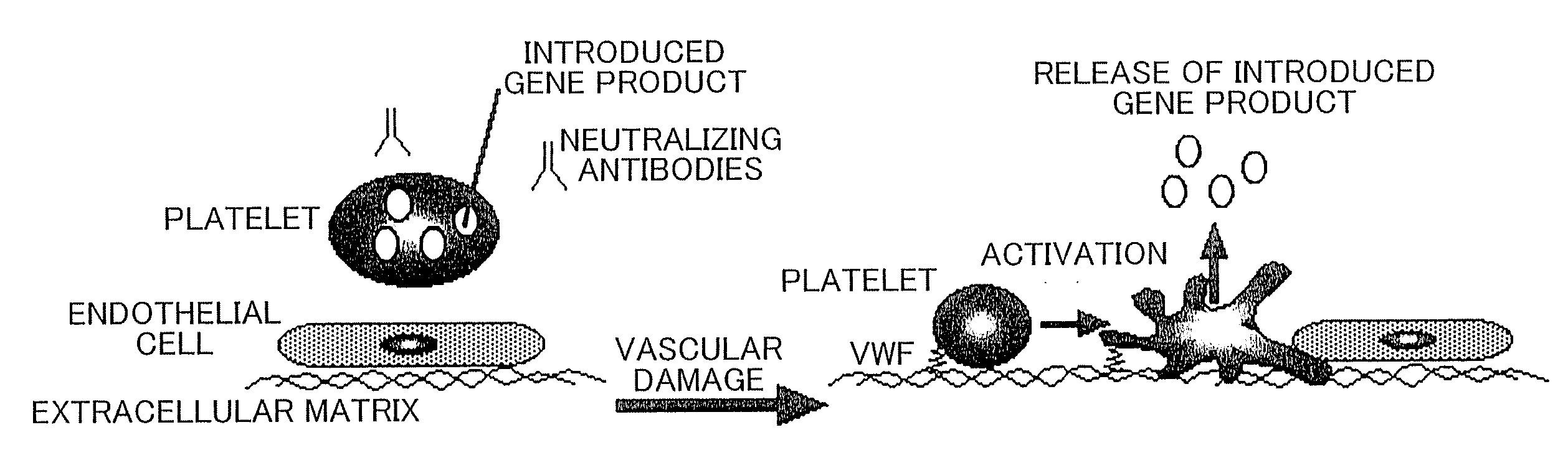
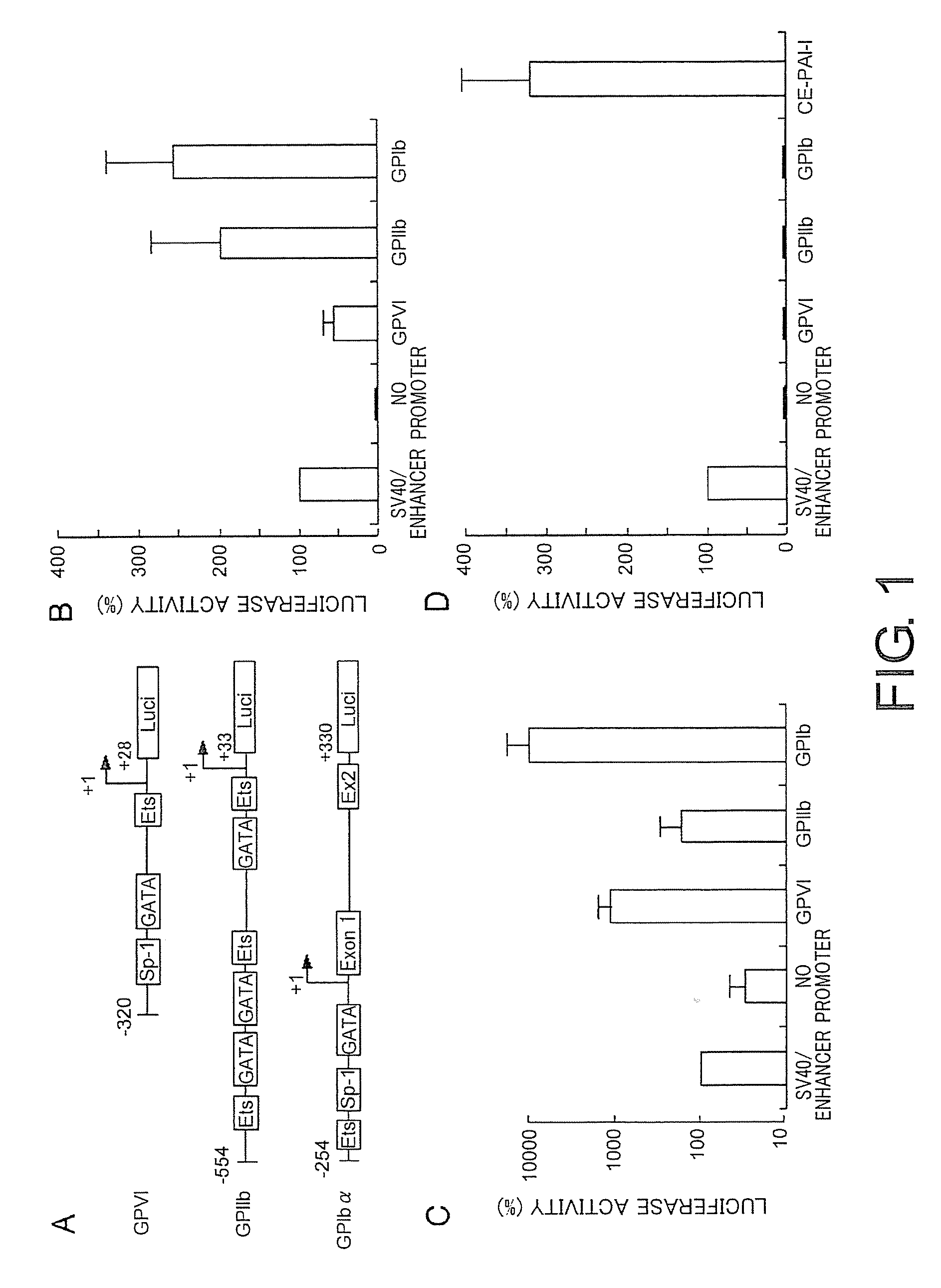
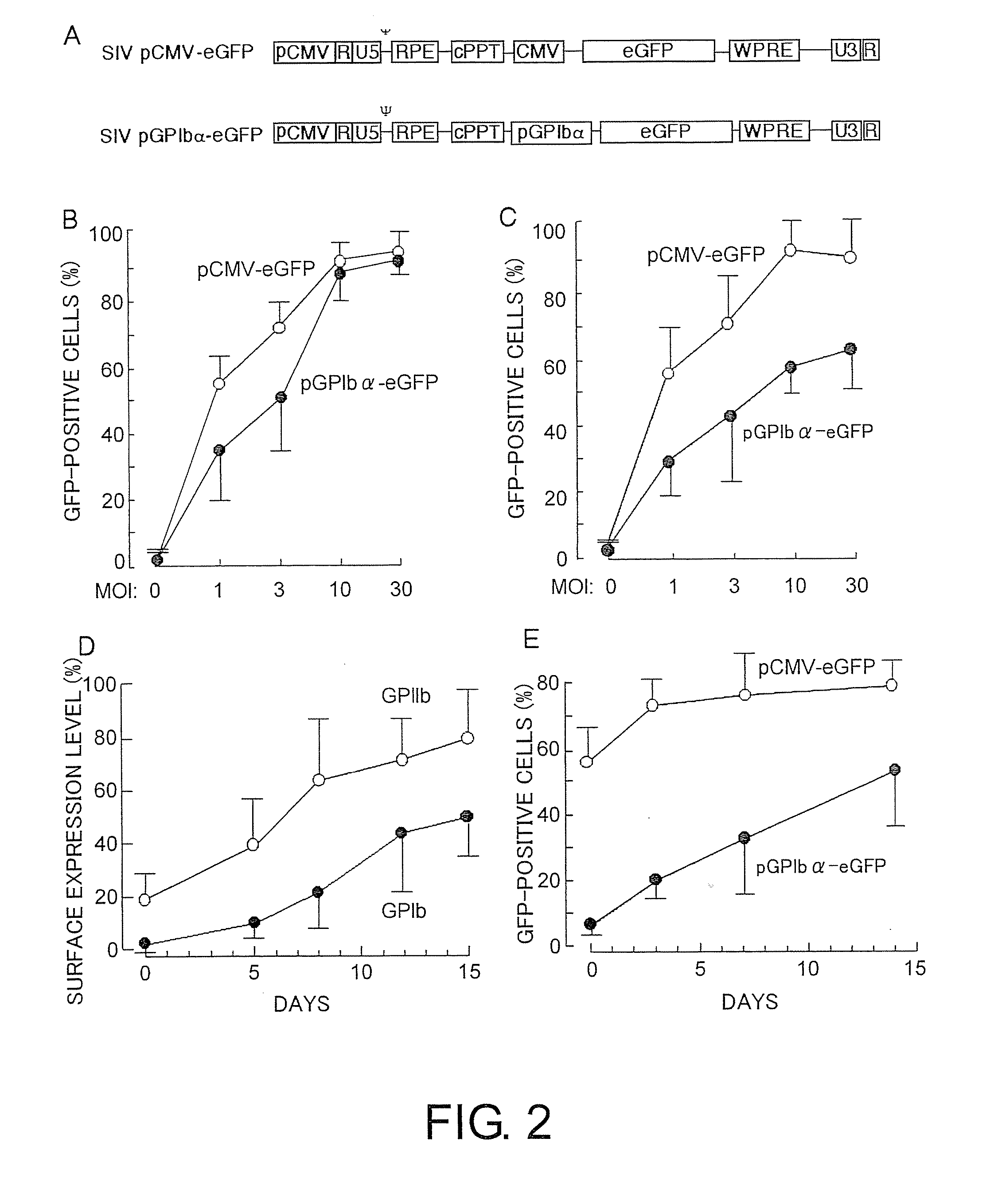
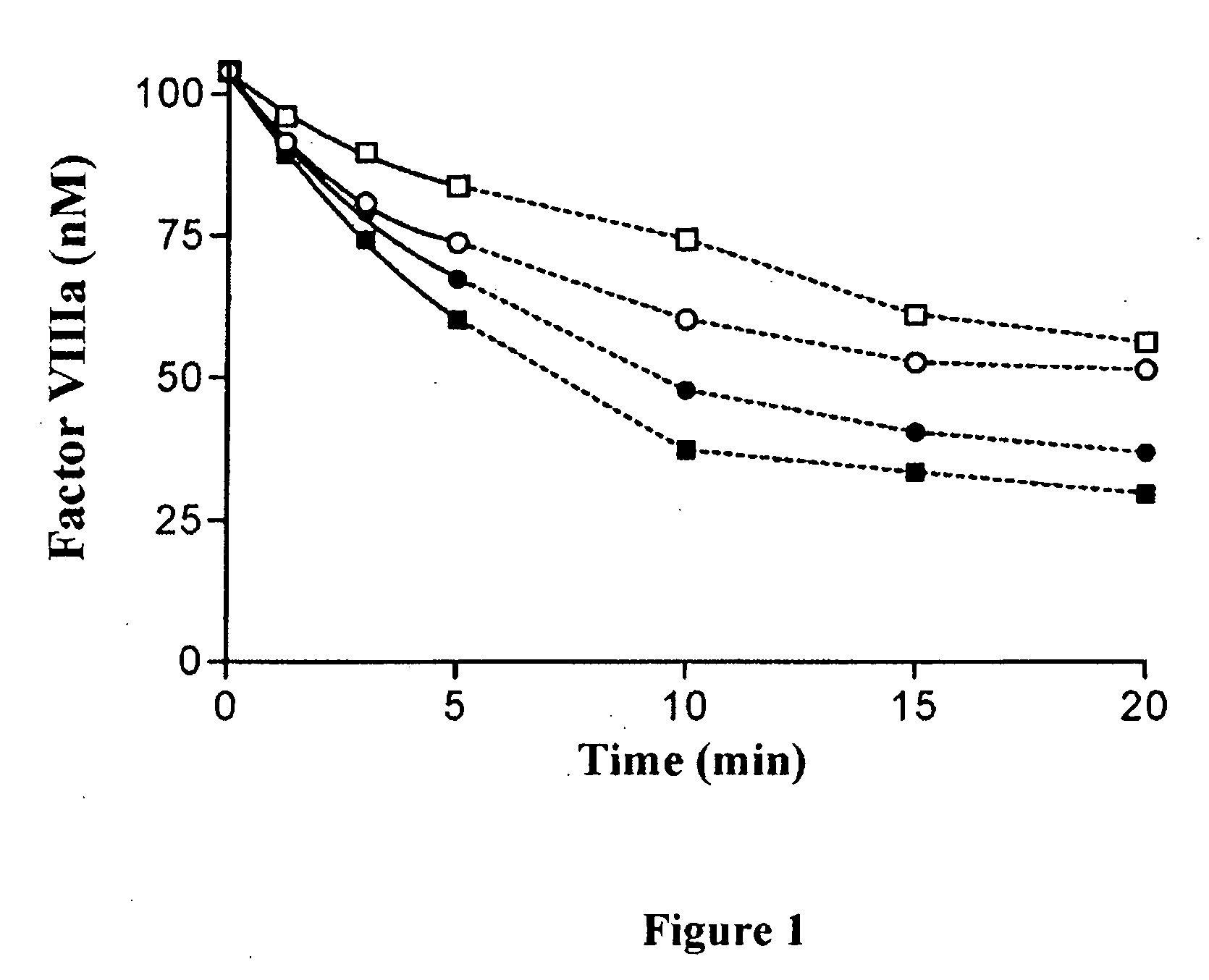
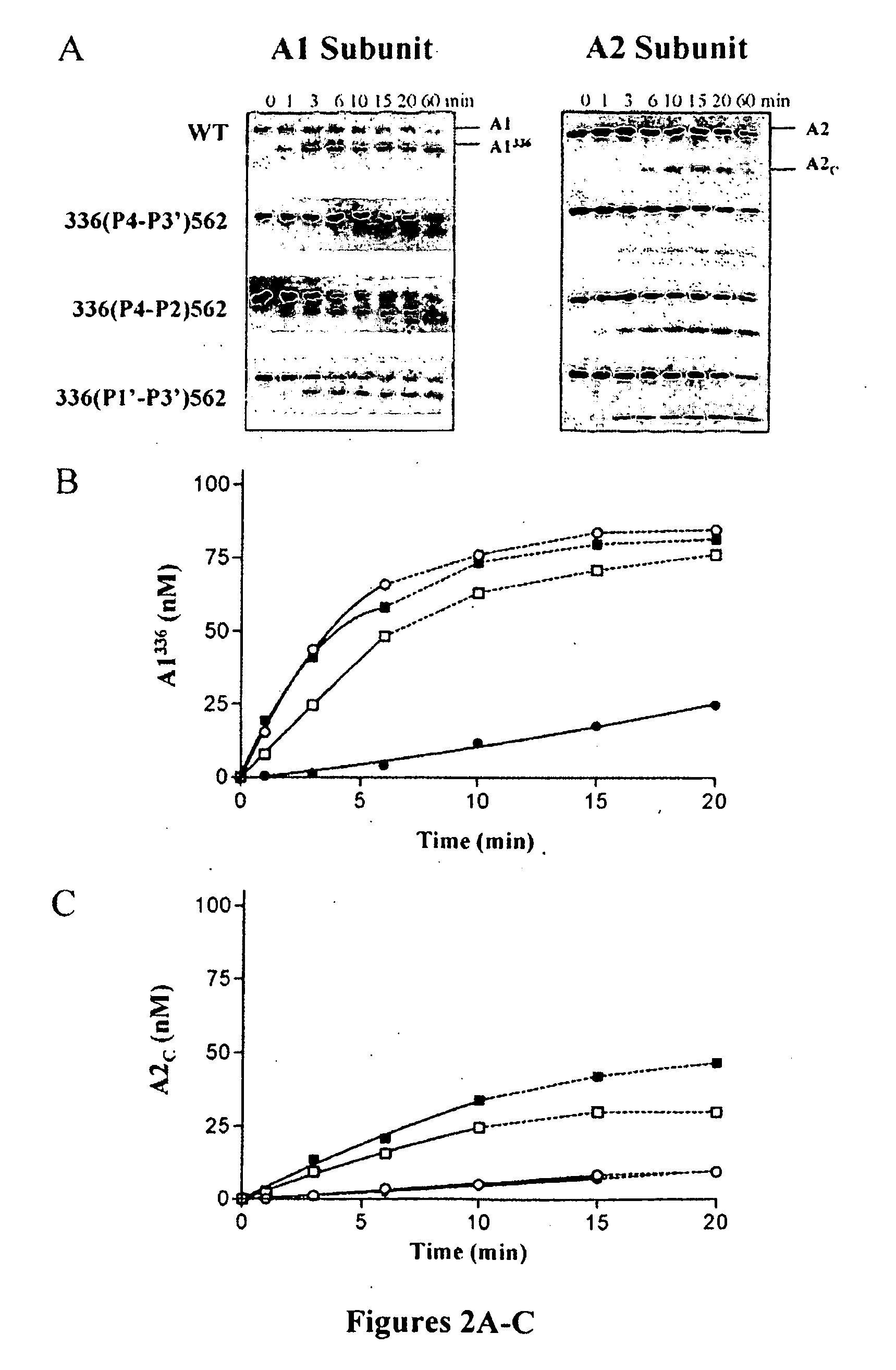
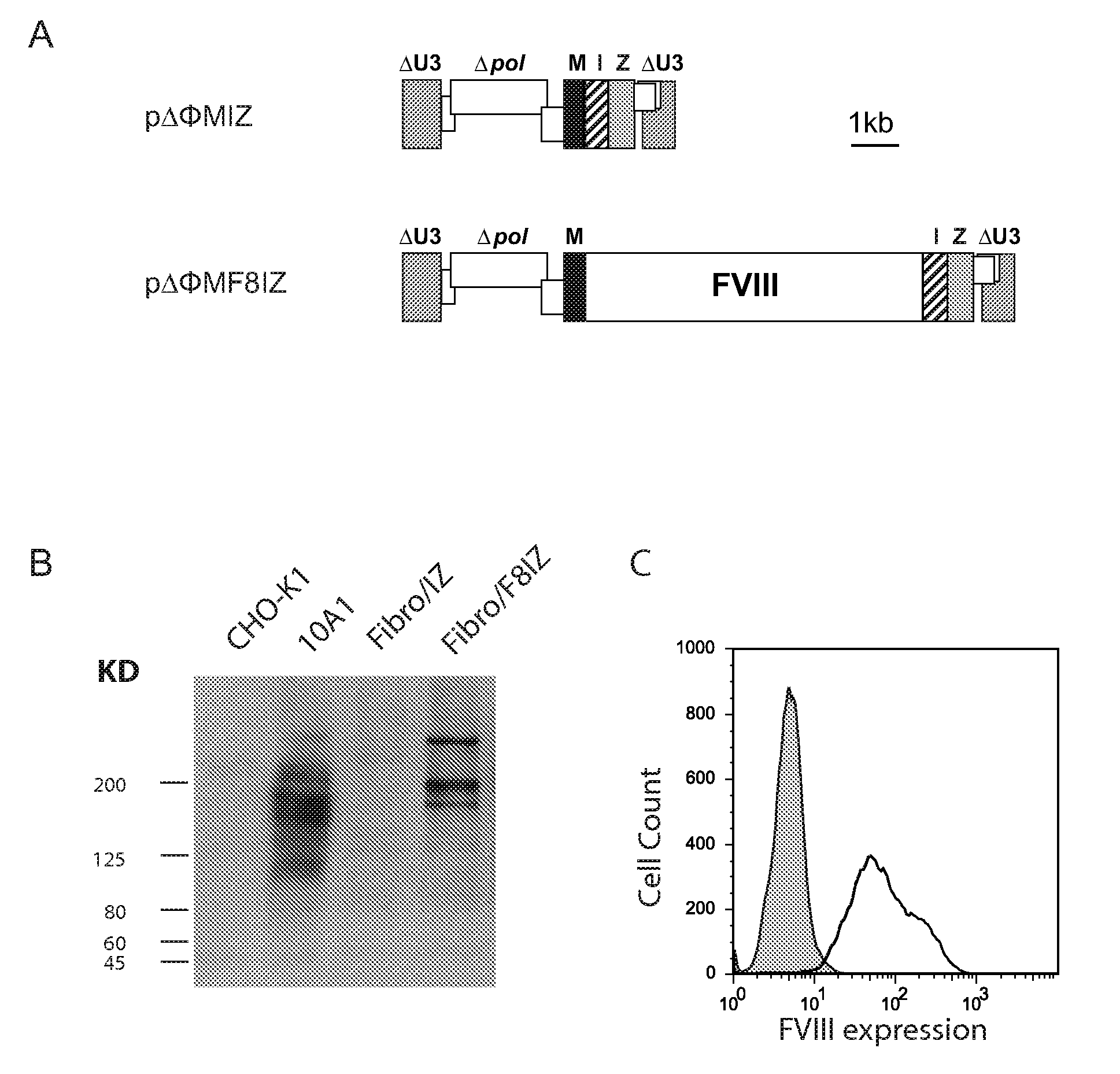
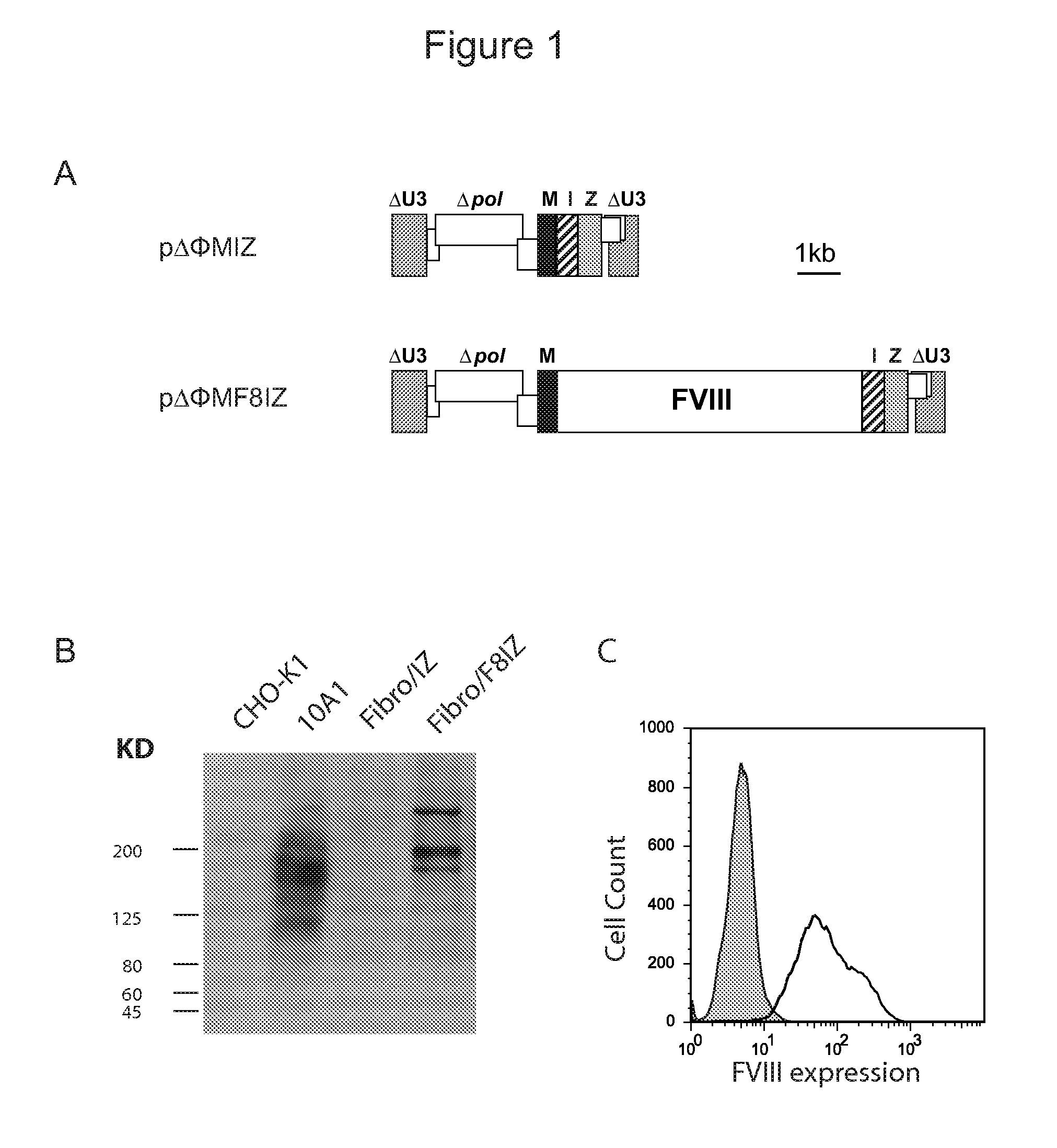
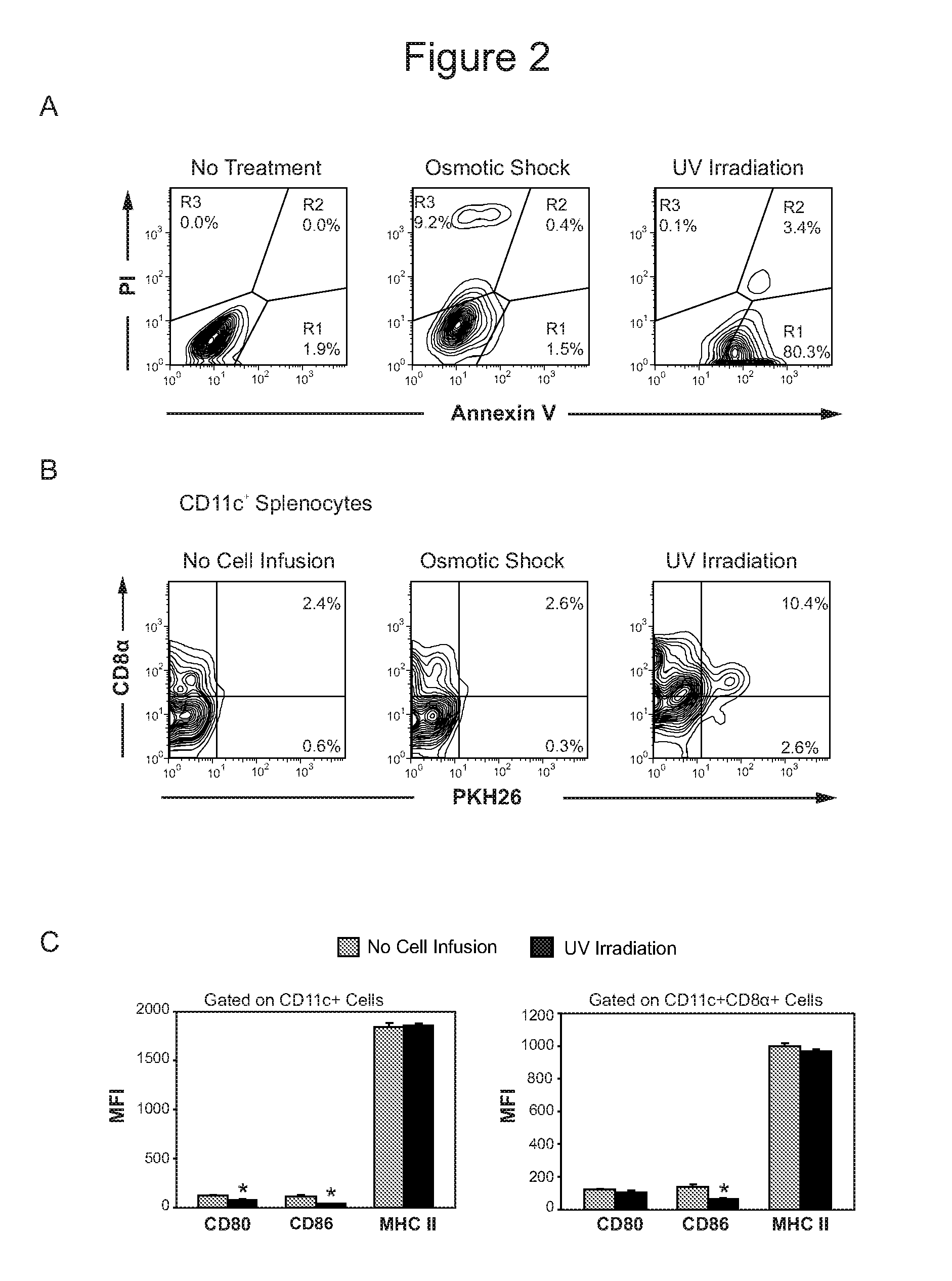
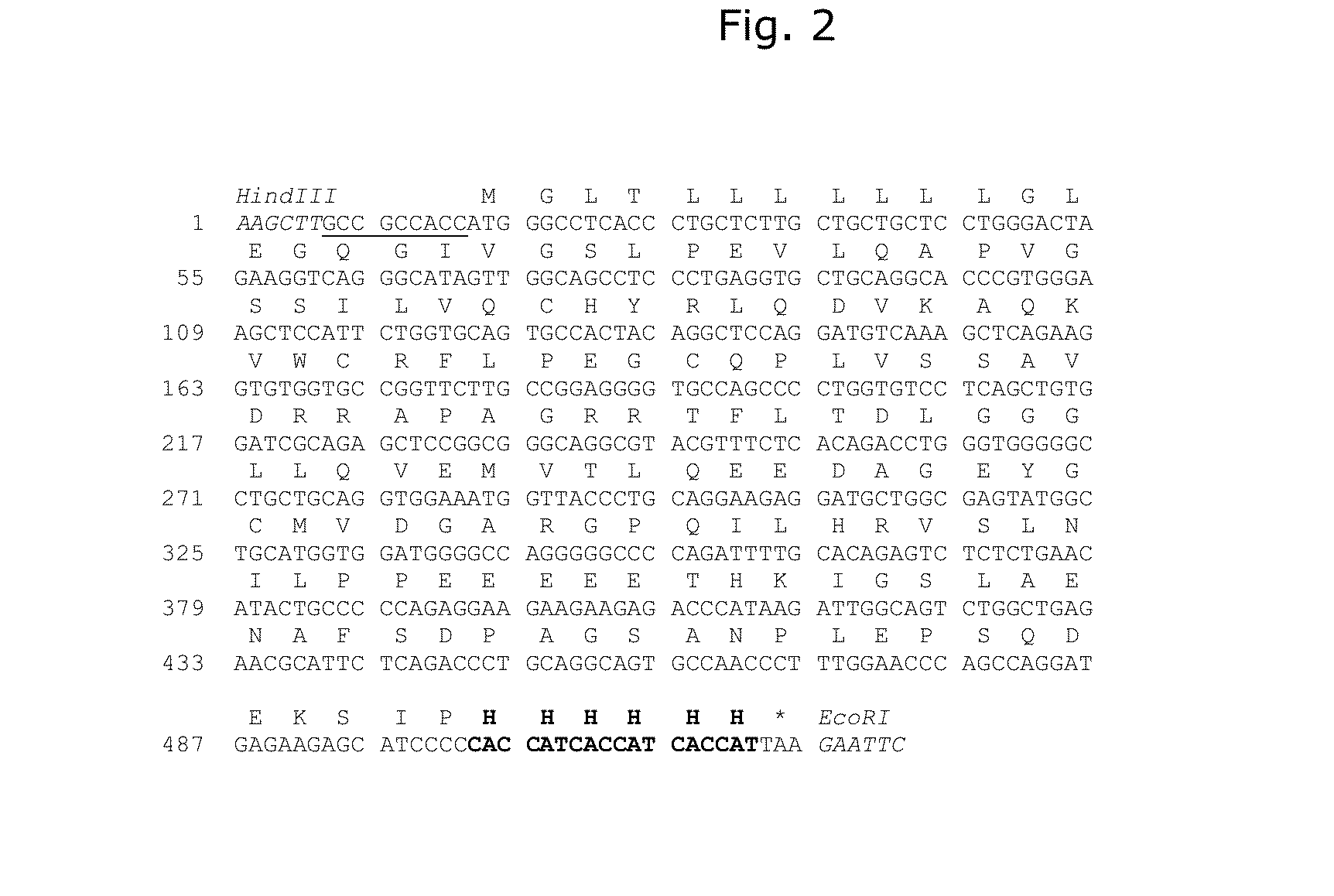
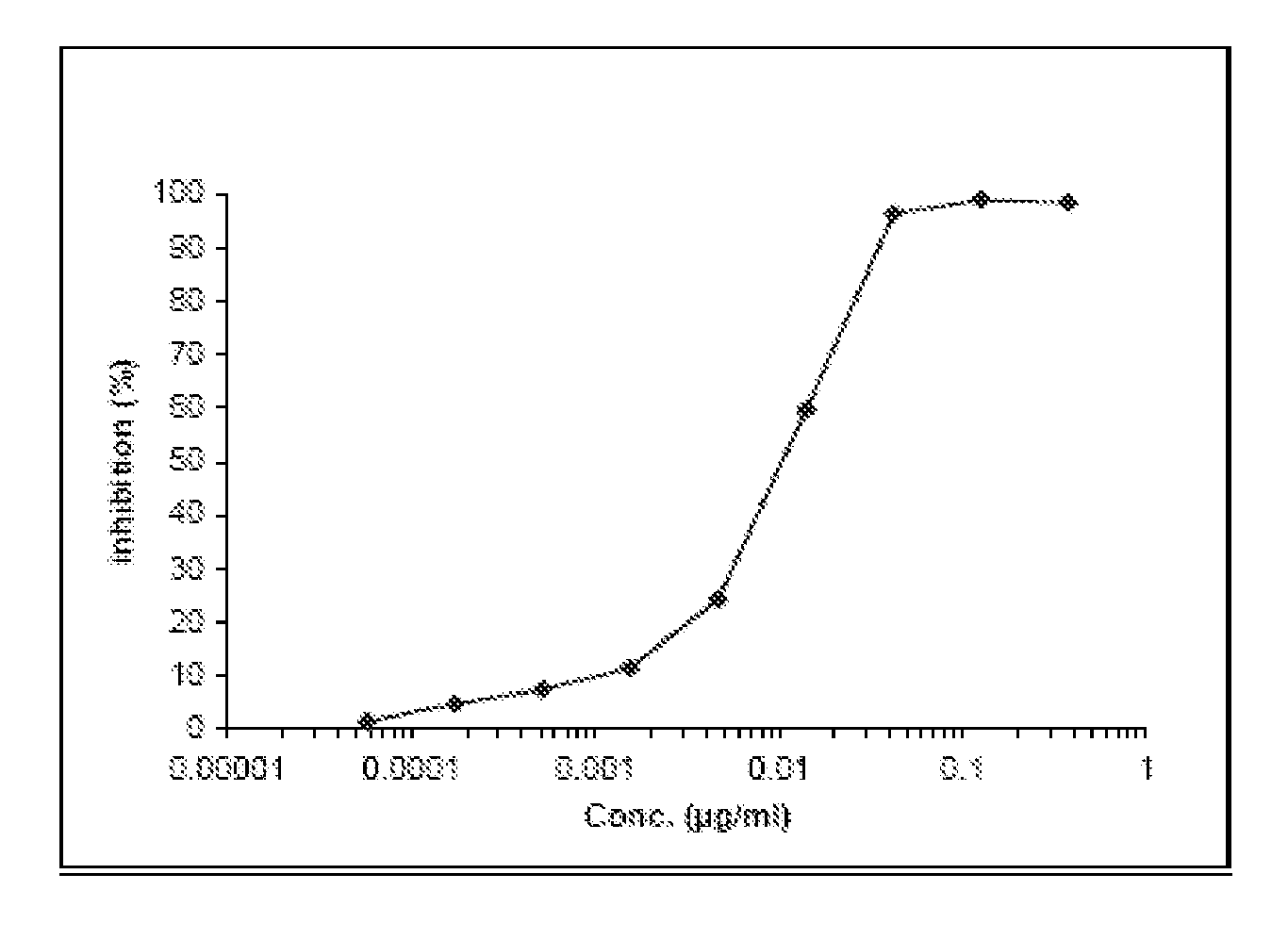
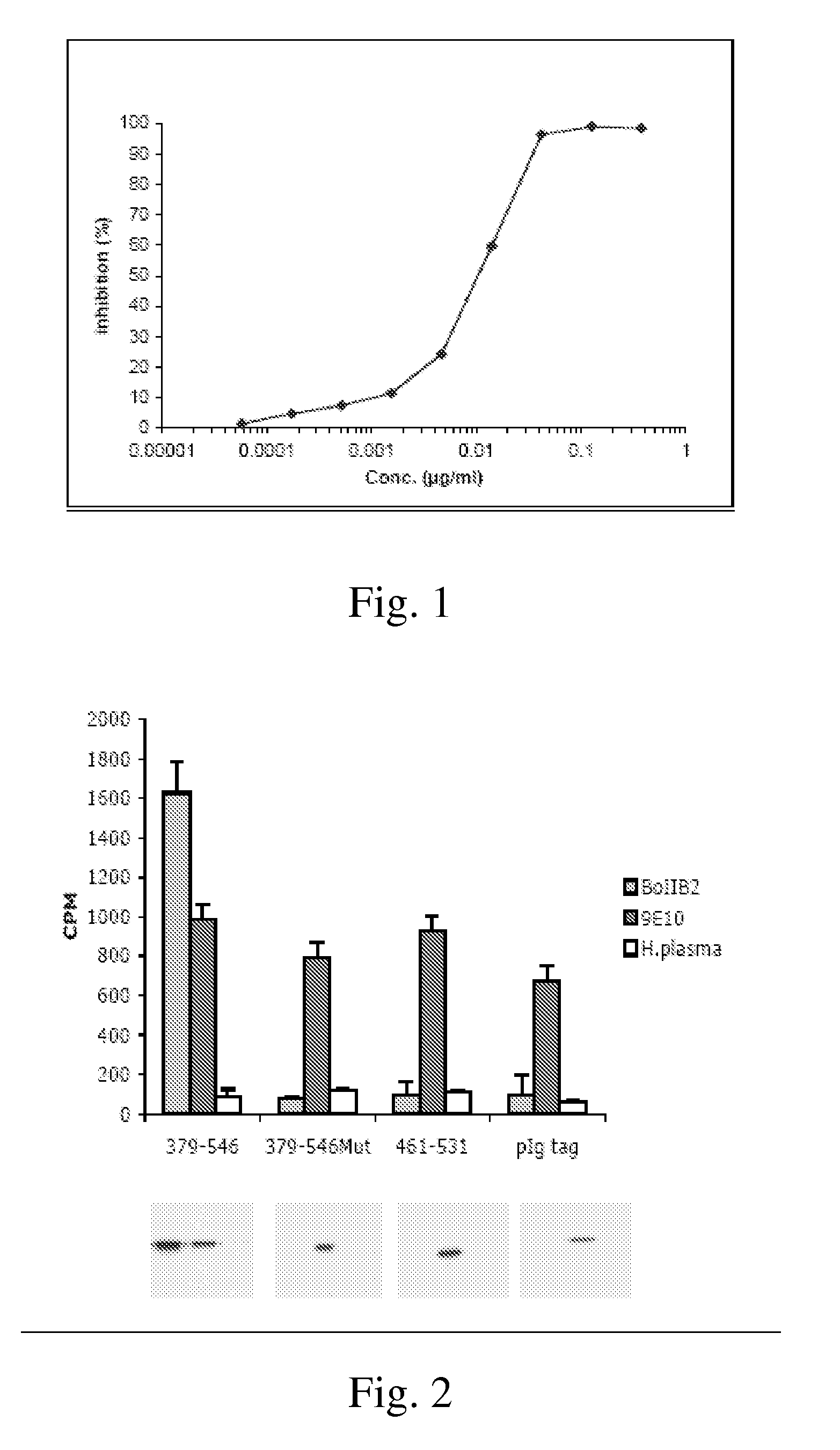
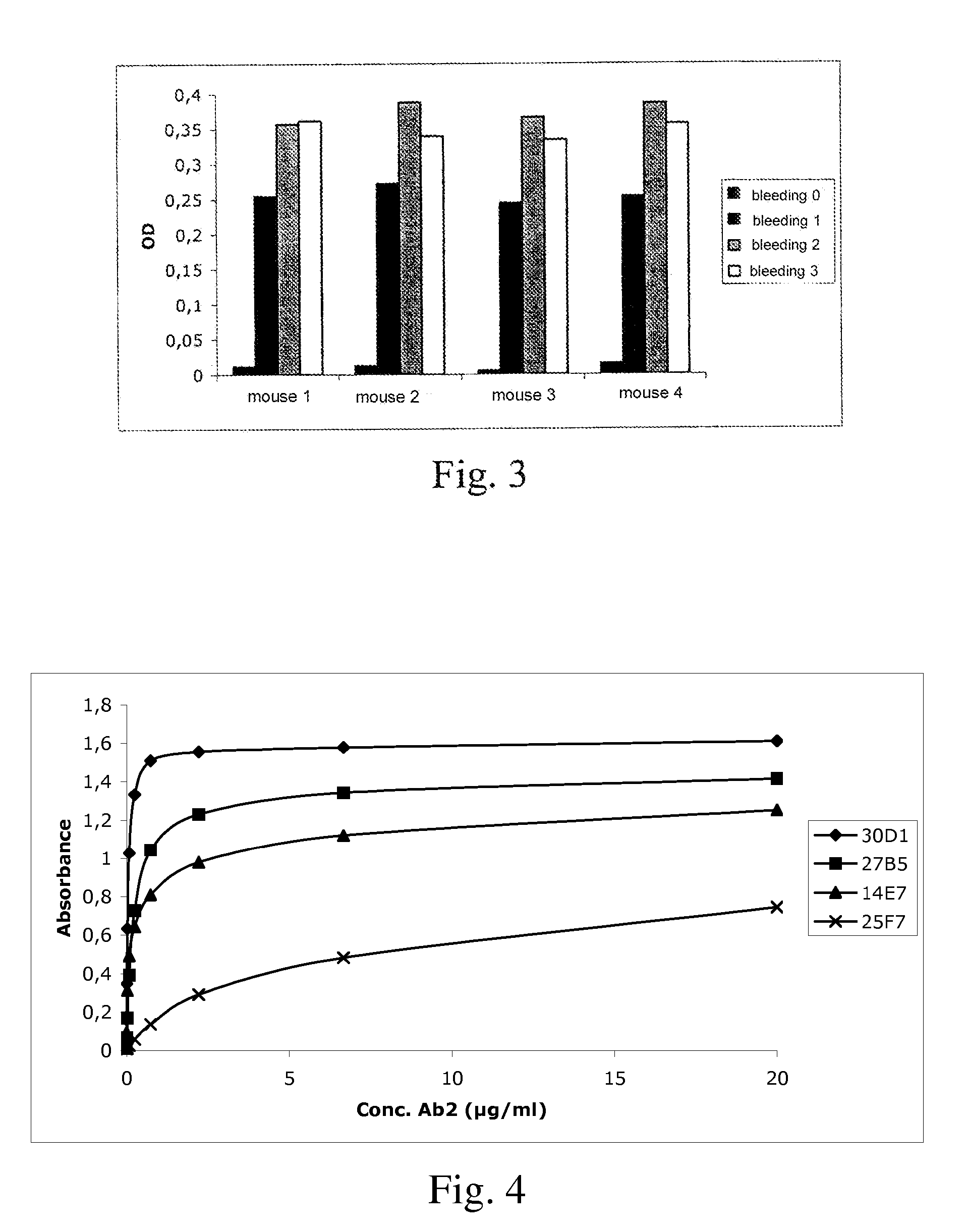
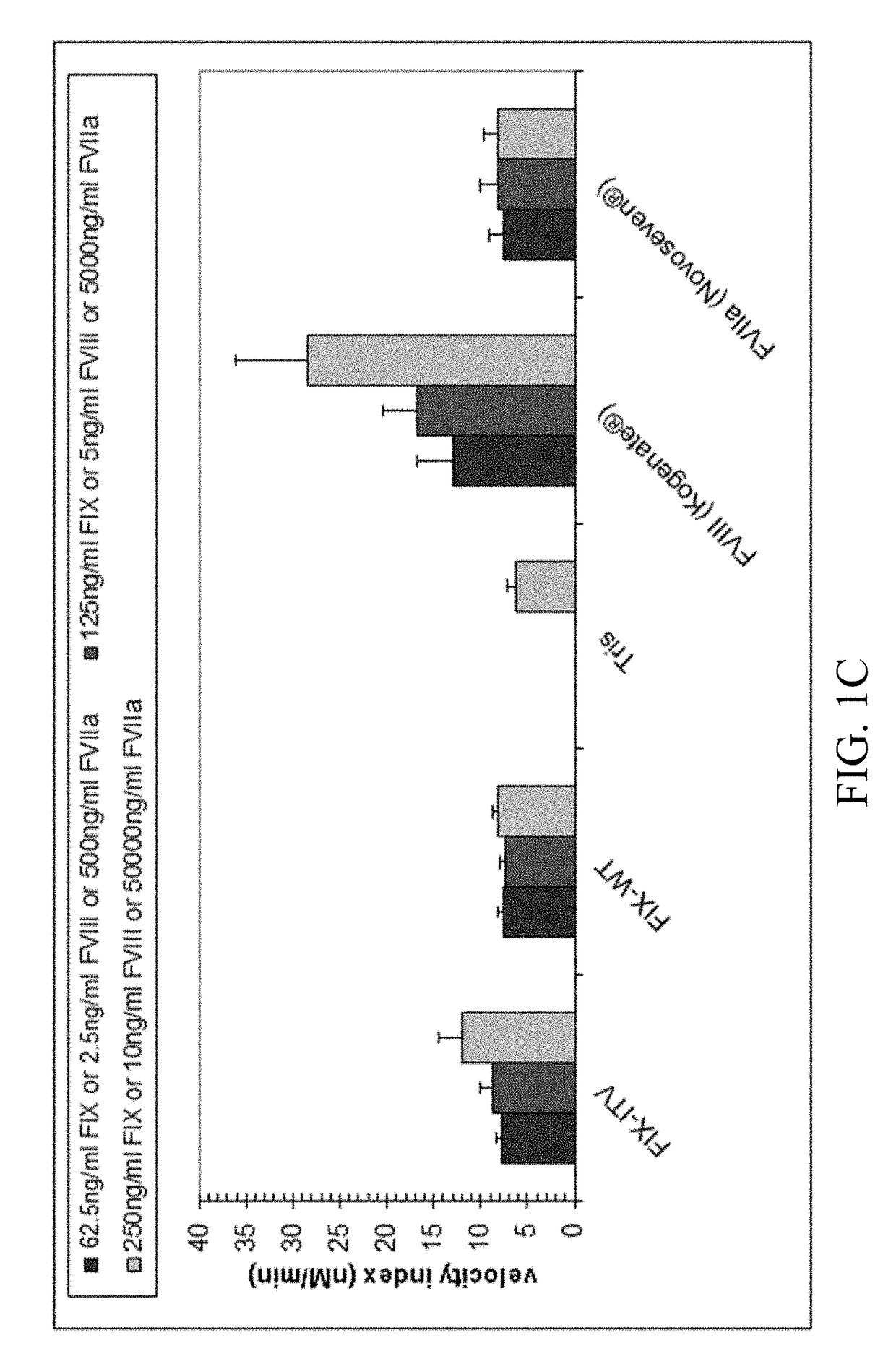
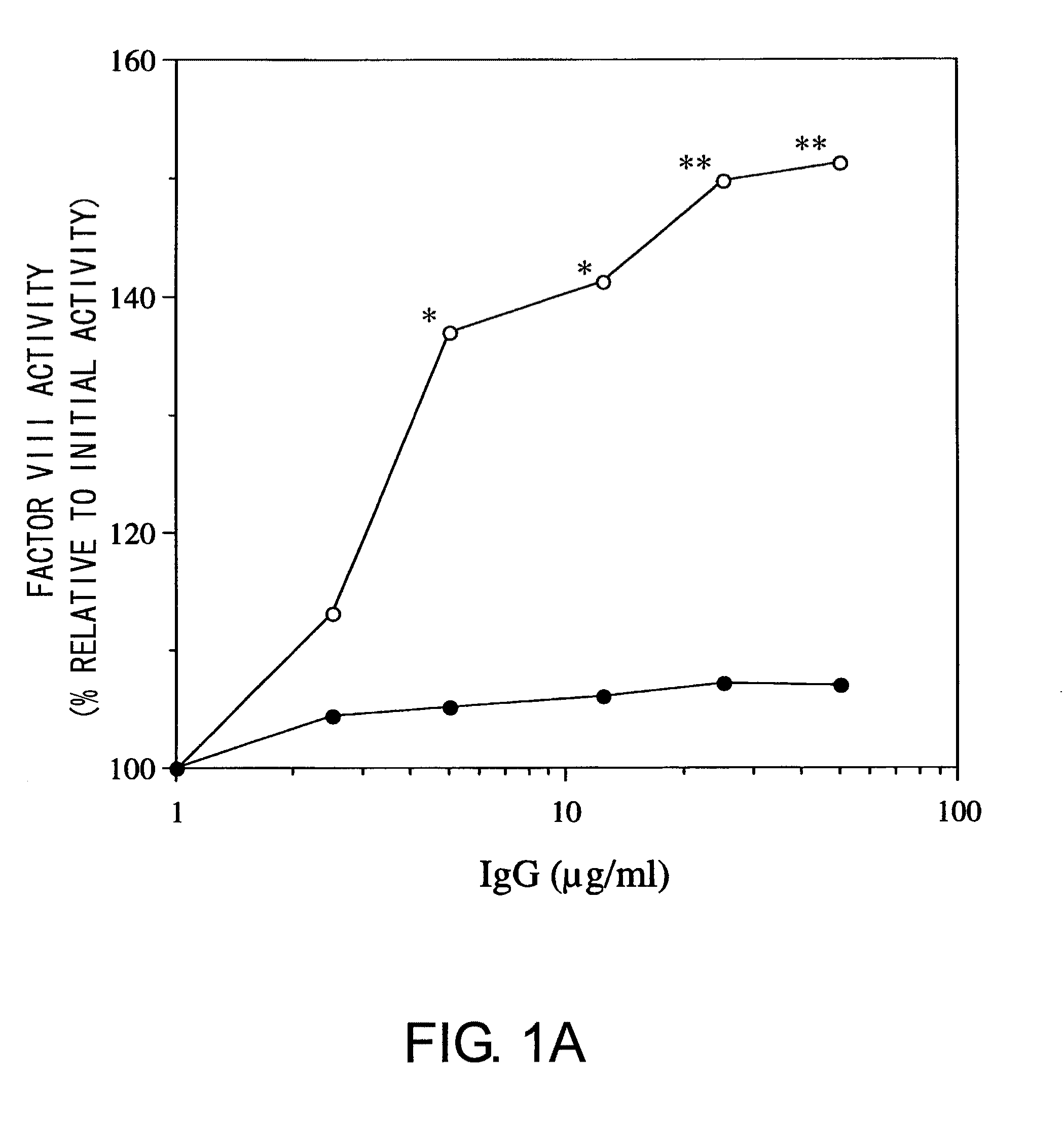
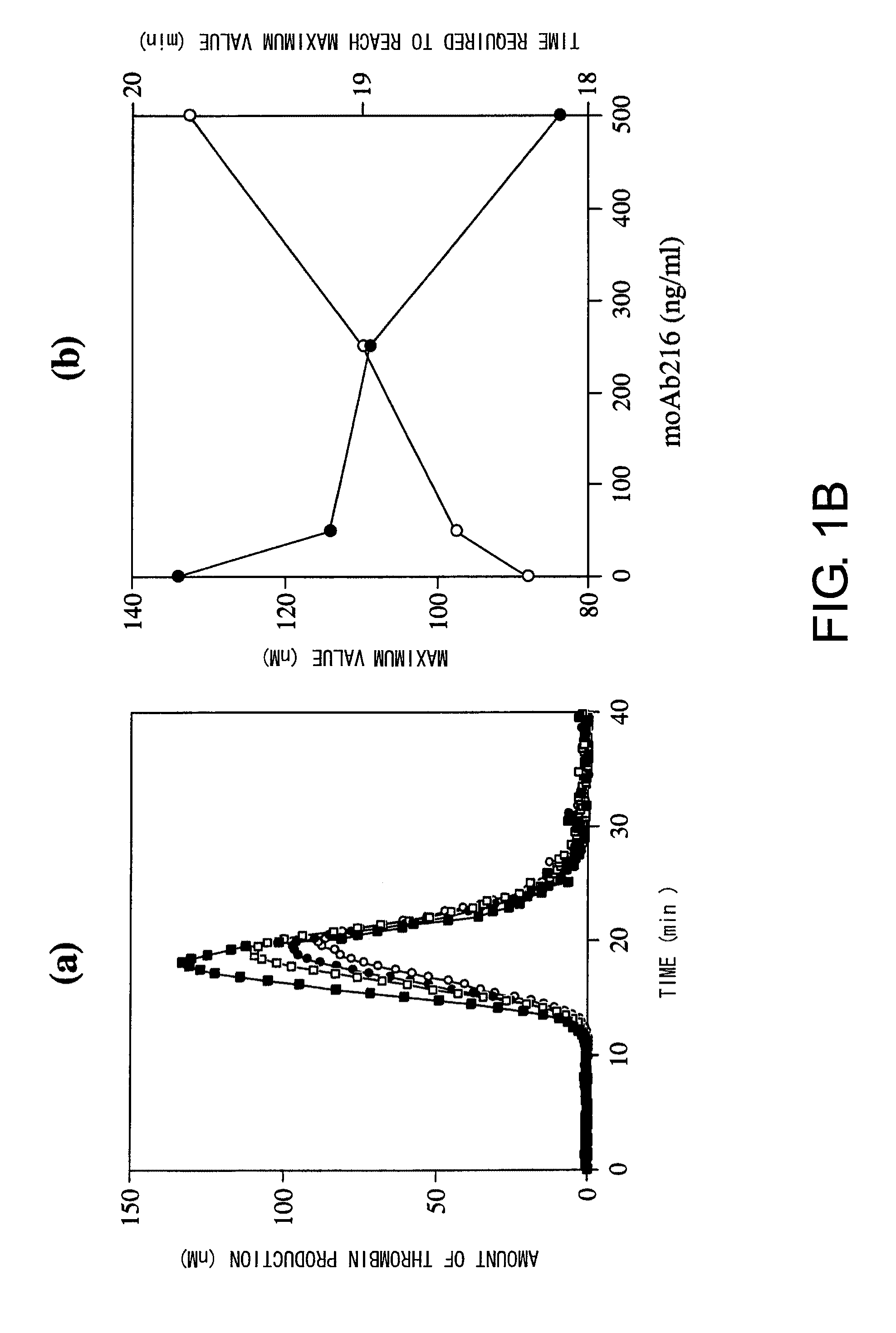
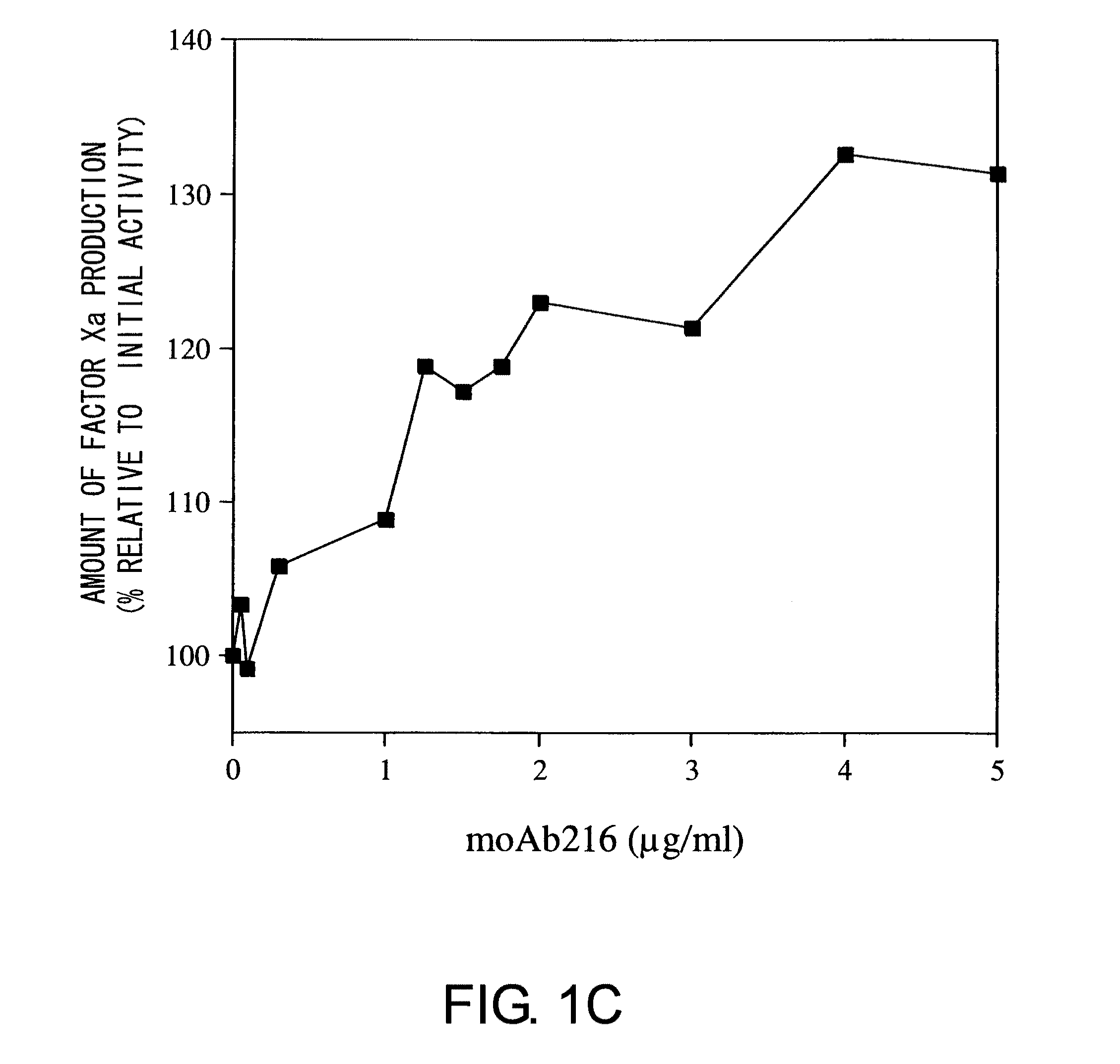
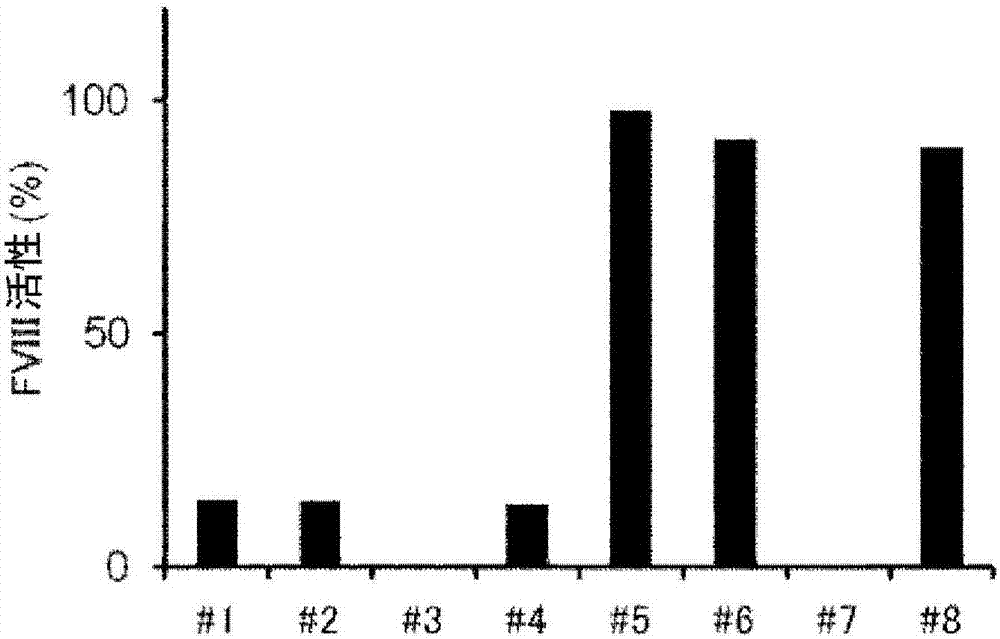
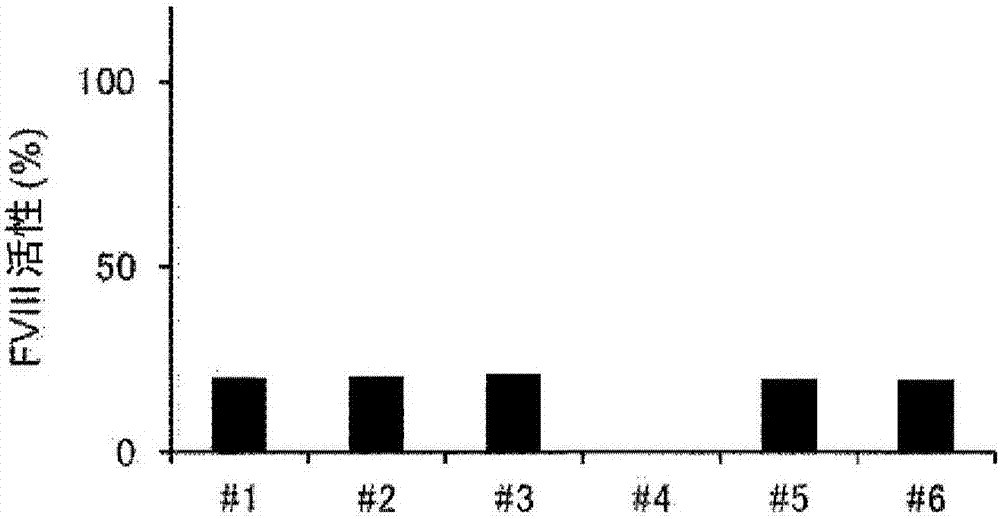
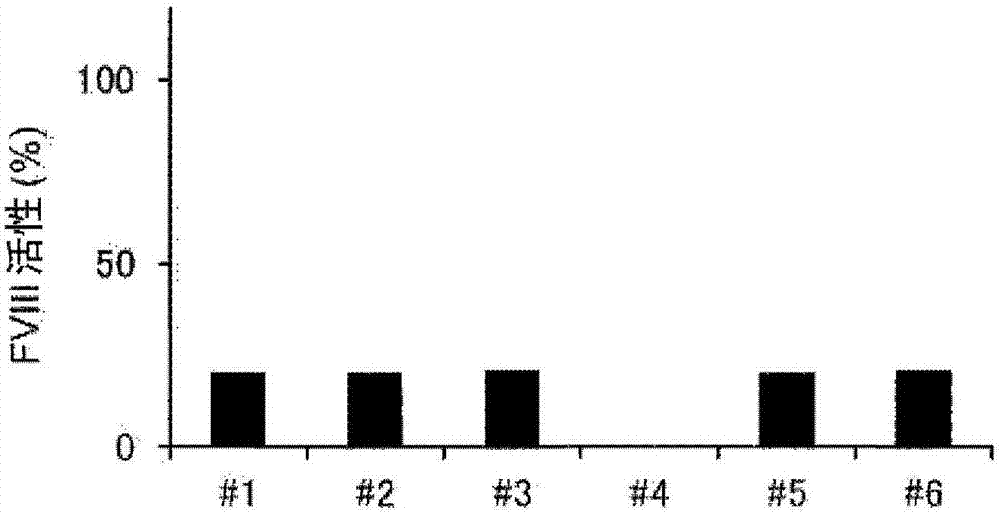
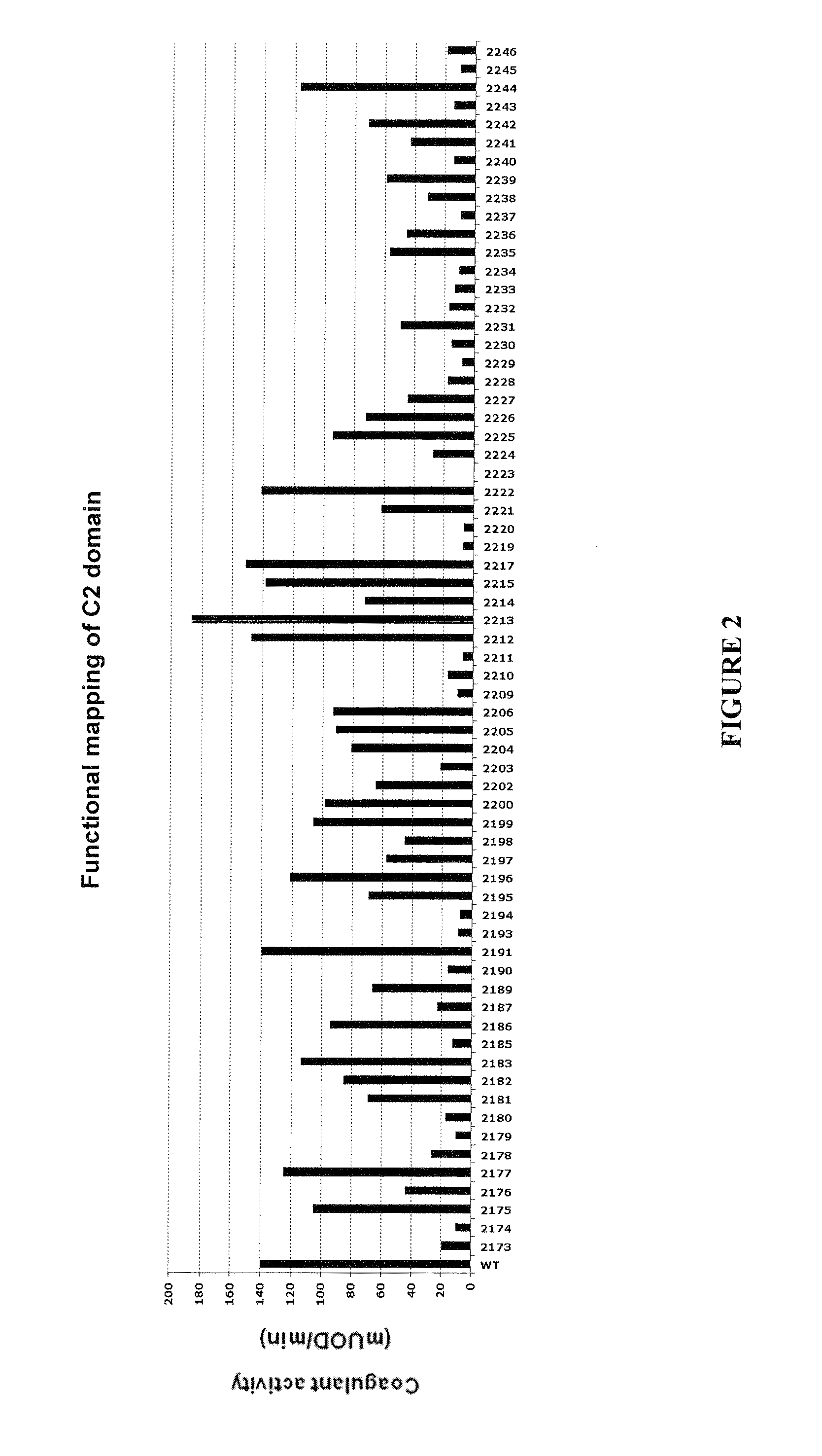
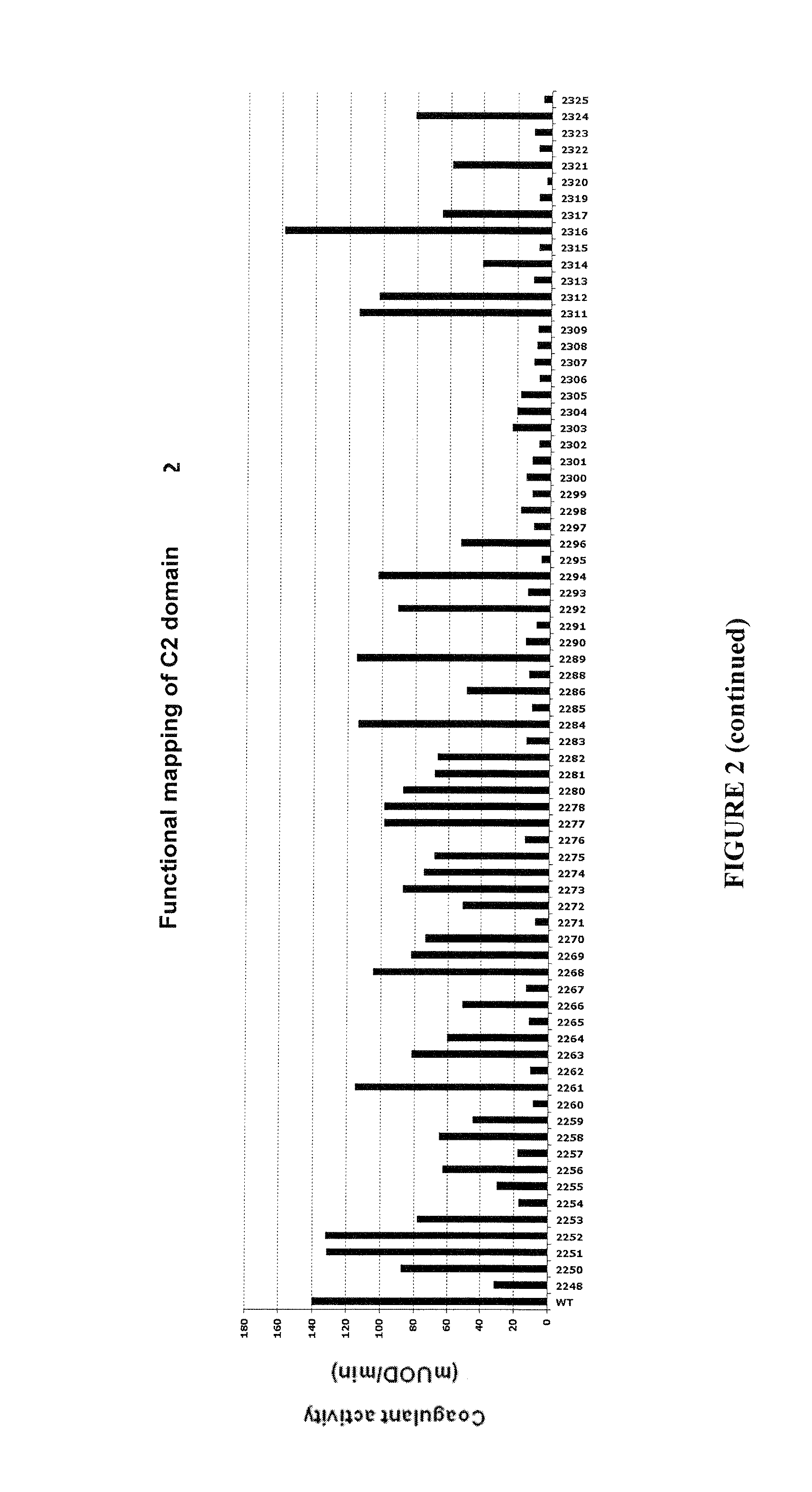
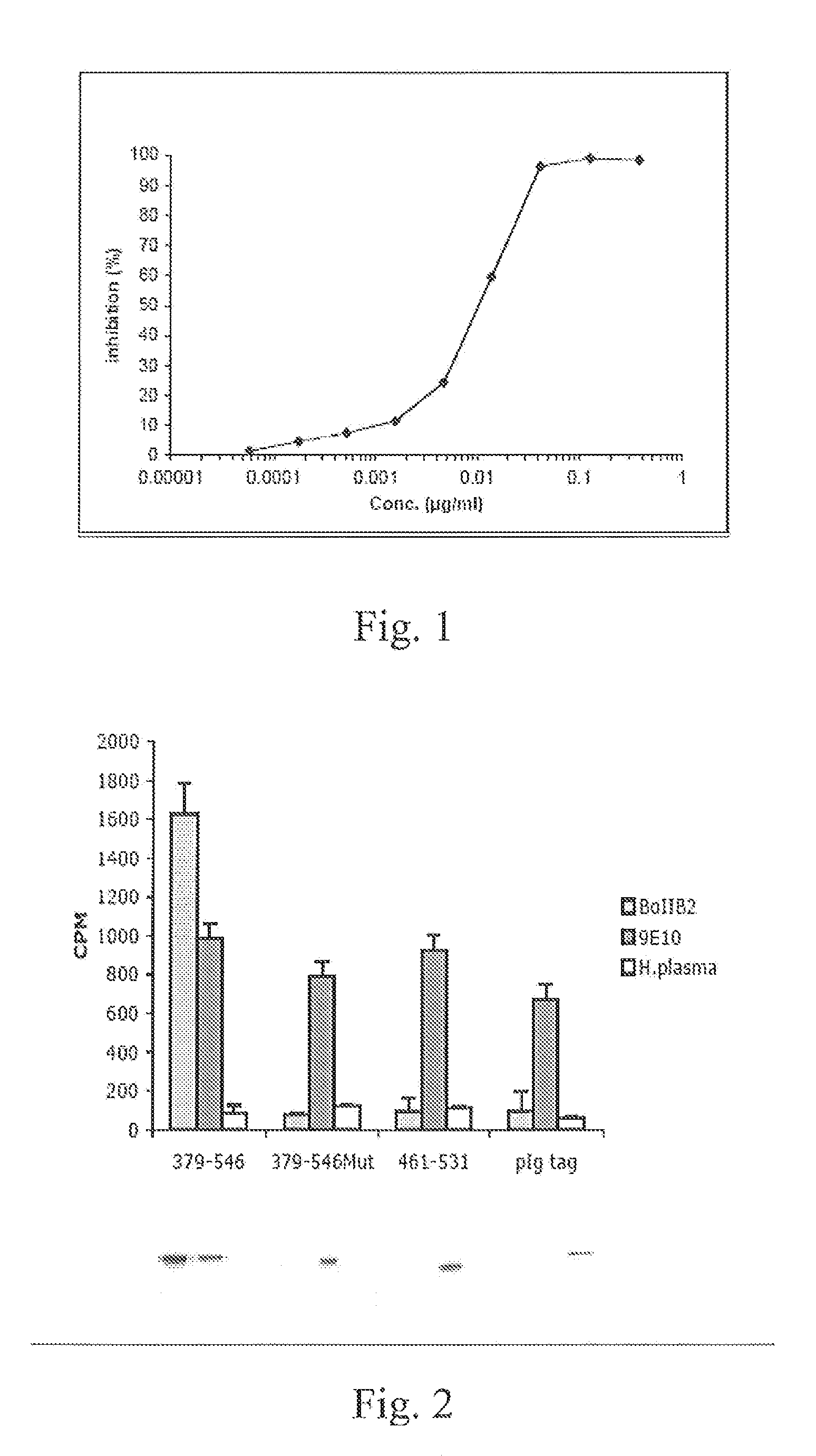
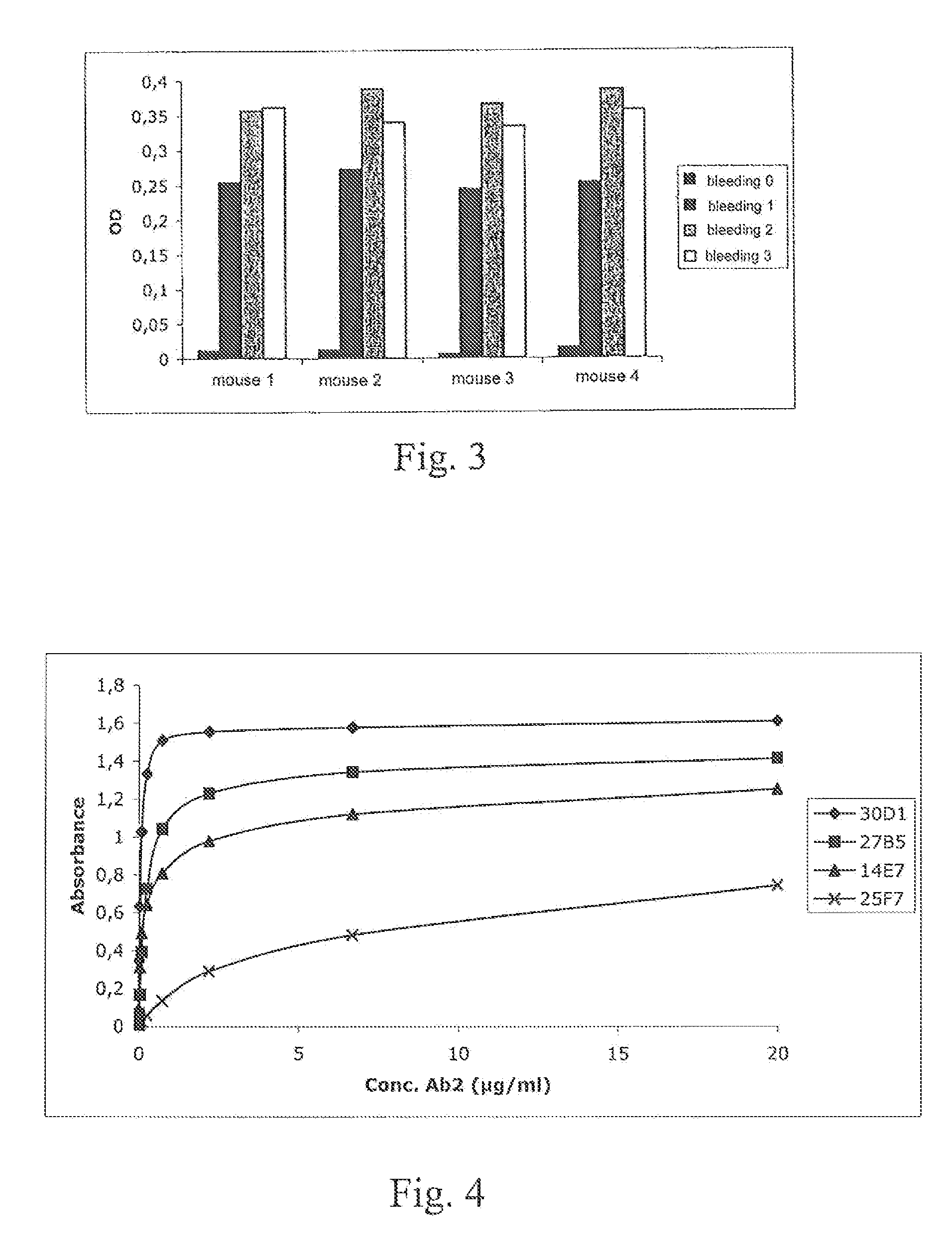
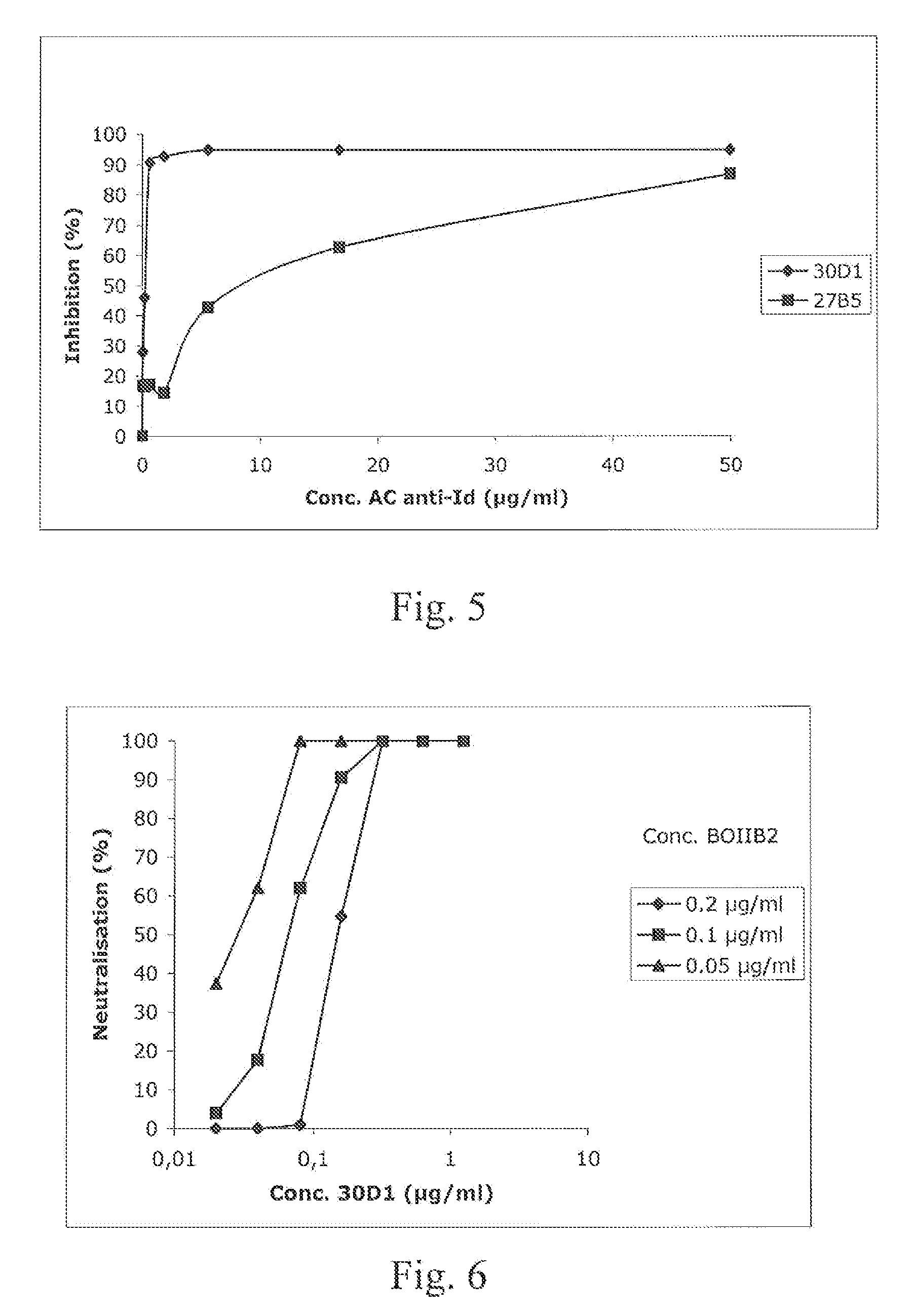
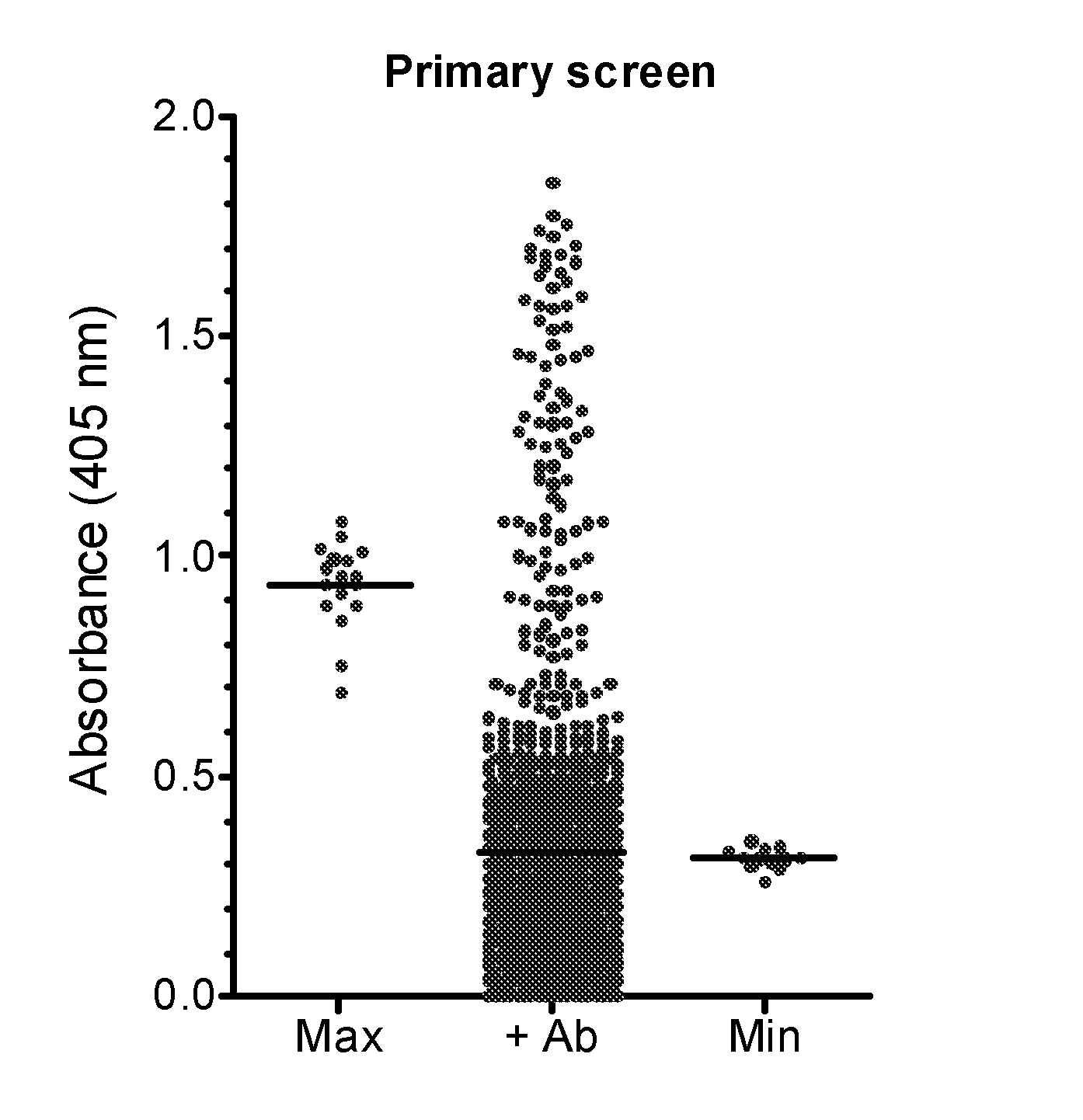
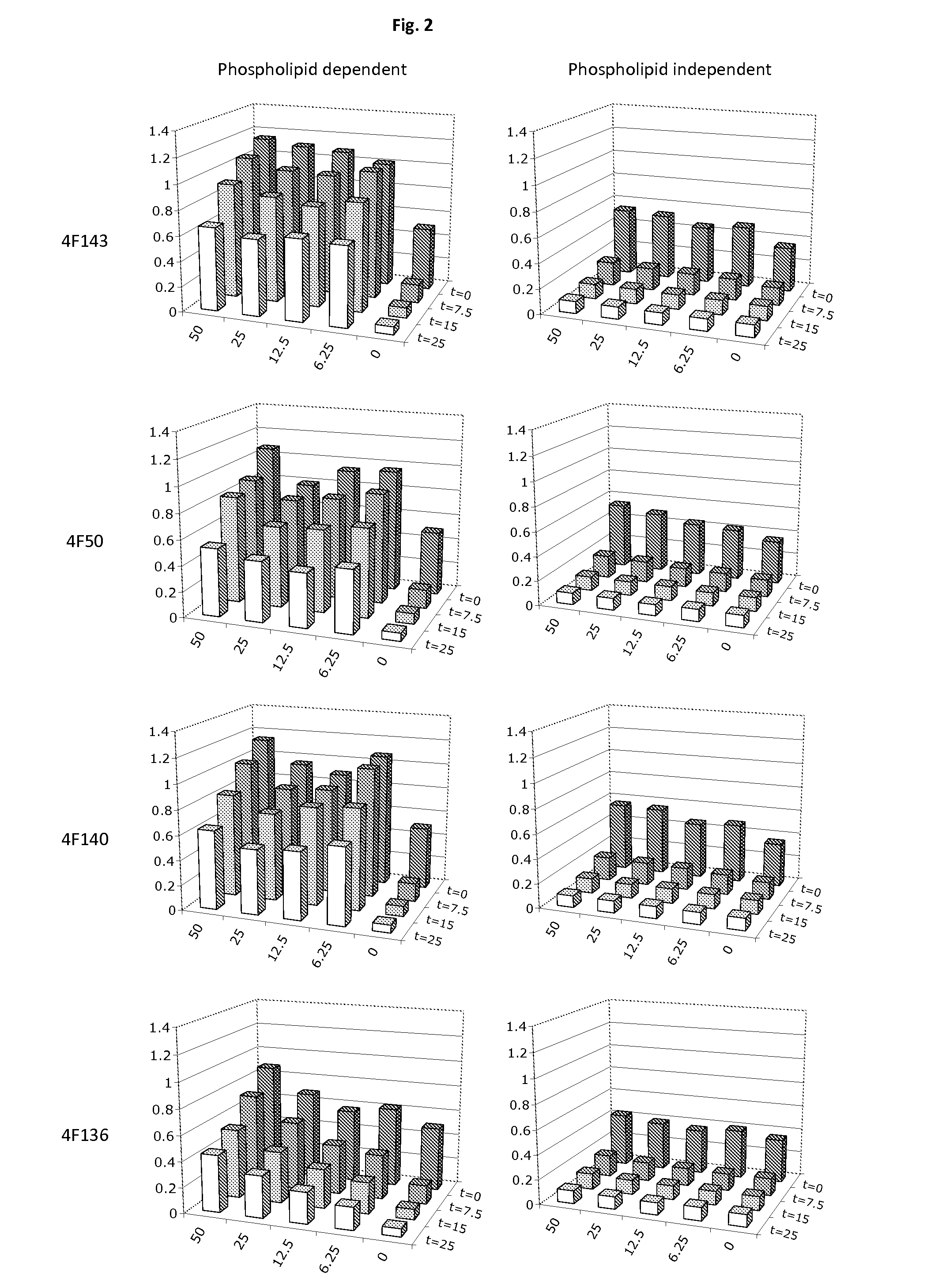
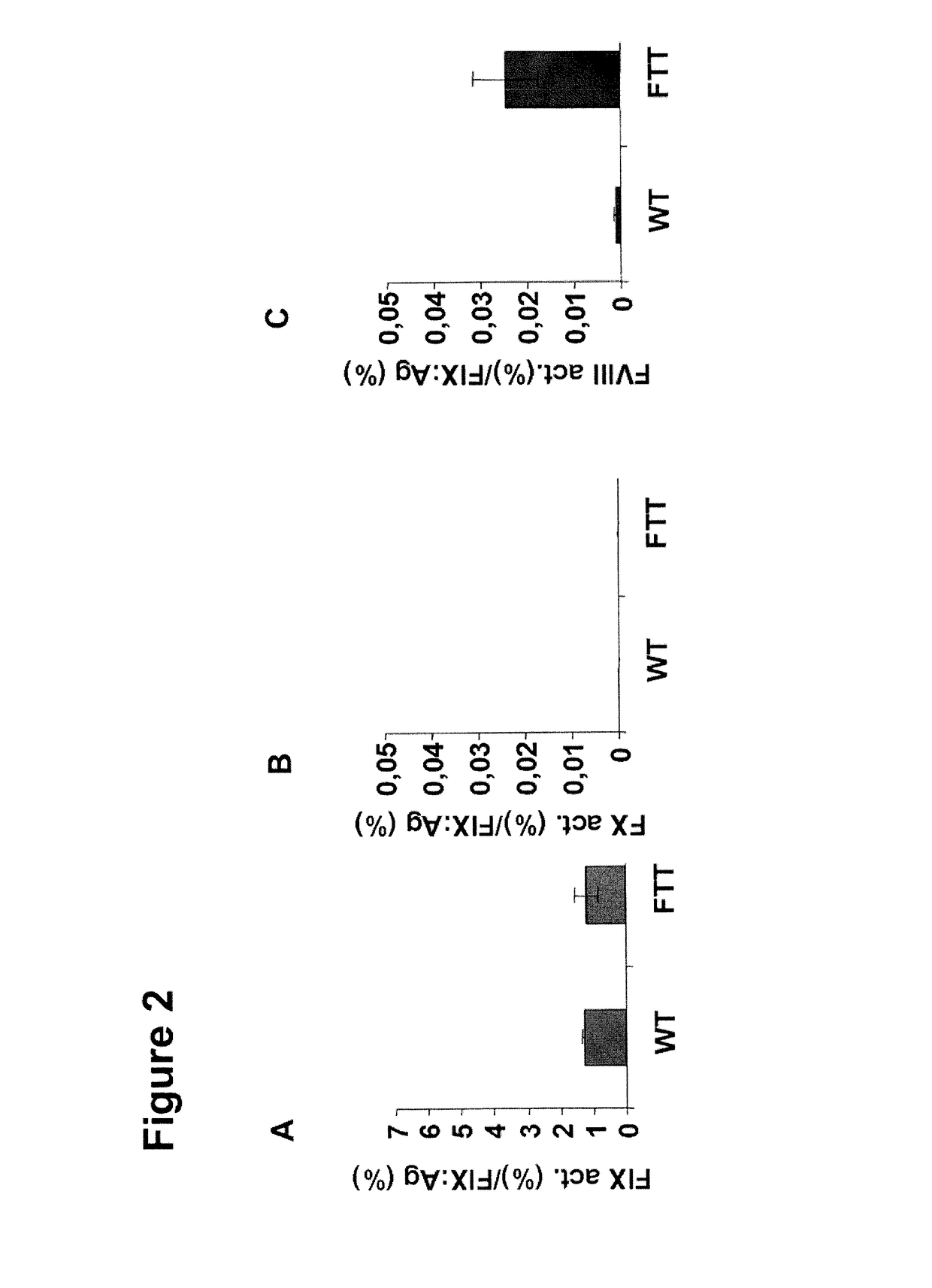
Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a
There is provided in accordance with the practice of this invention a demannosylated Factor VIII, the immunogenicity of which is substantially decreased or abolished in Human. The modified factor VIII is disclosed together with the modified amino acid sequence, changed by at least one substitution. The modified factor VIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory anti-FVIII antibodies.
Owner:LFB BIOTECH +1
Demannosylated recombinant factor VIII for the treatment of patients with haemophilia A
There is provided in accordance with the practice of this invention a demannosylated Factor VIII, the immunogenicity of which is substantially decreased or abolished in Human. The modified factor VIII is disclosed together with the modified amino acid sequence, changed by at least one substitution. The modified factor VIII is useful for hemophiliacs, either to avoid or prevent the action of inhibitory anti-FVIII antibodies.
Owner:LFB BIOTECH +1
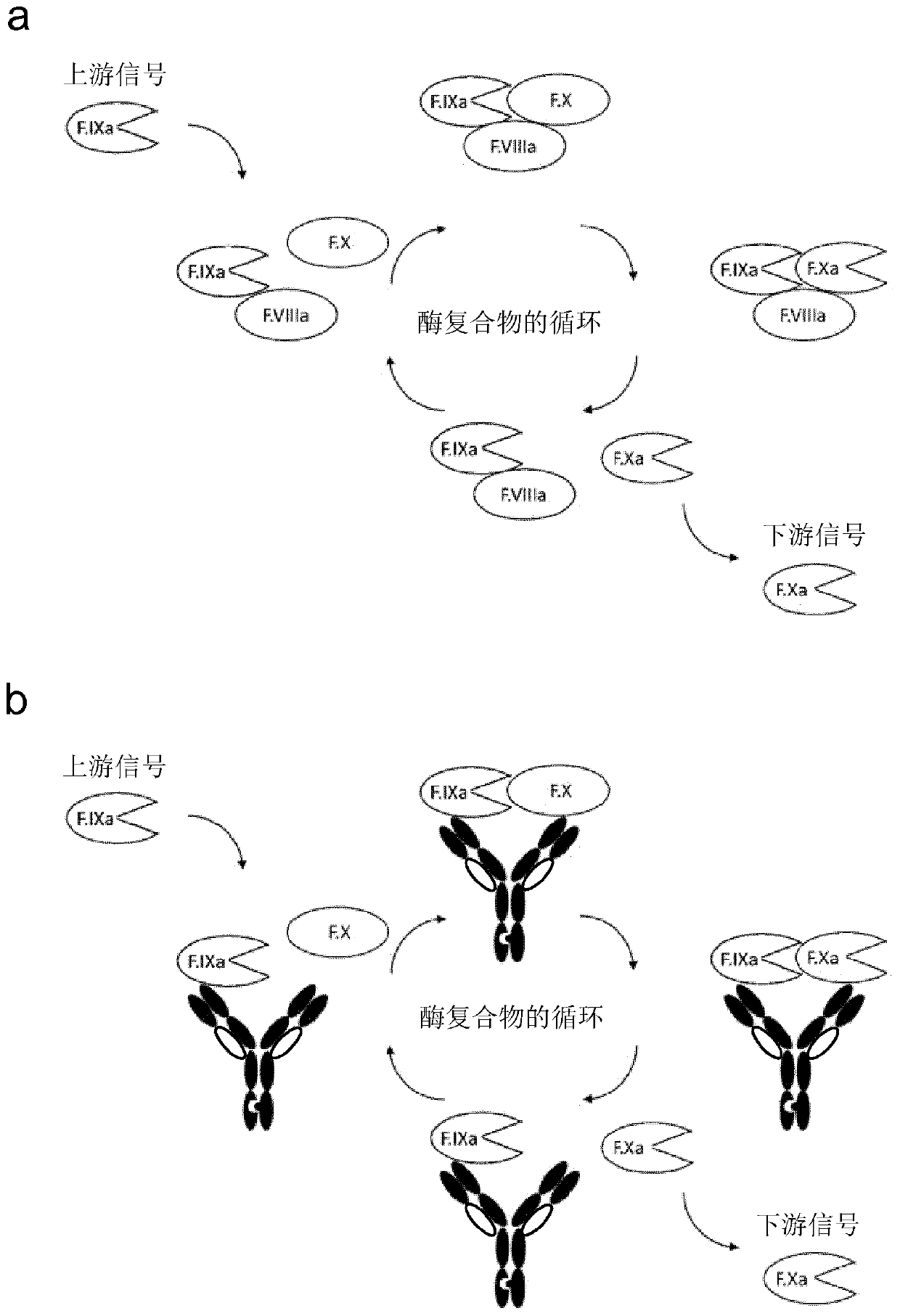
Bispecific antibodies for factor ix and factor x
PendingCN110753704AReduce dosing frequencyEasy for subcutaneous injectionImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsAntiendomysial antibodiesBispecific antibody
Bispecific antigen binding molecules (e.g., antibodies) that bind blood clotting factors, factor IXa (FIXa) and factor X (FX), and enhance the FIXa-catalysed activation of FX to FXa. Use of the bispecific antigen binding molecules is to control bleeding, by replacing natural cofactor FVIIIa which is deficient in patients with haemophilia A.
Owner:KIMAB LTD
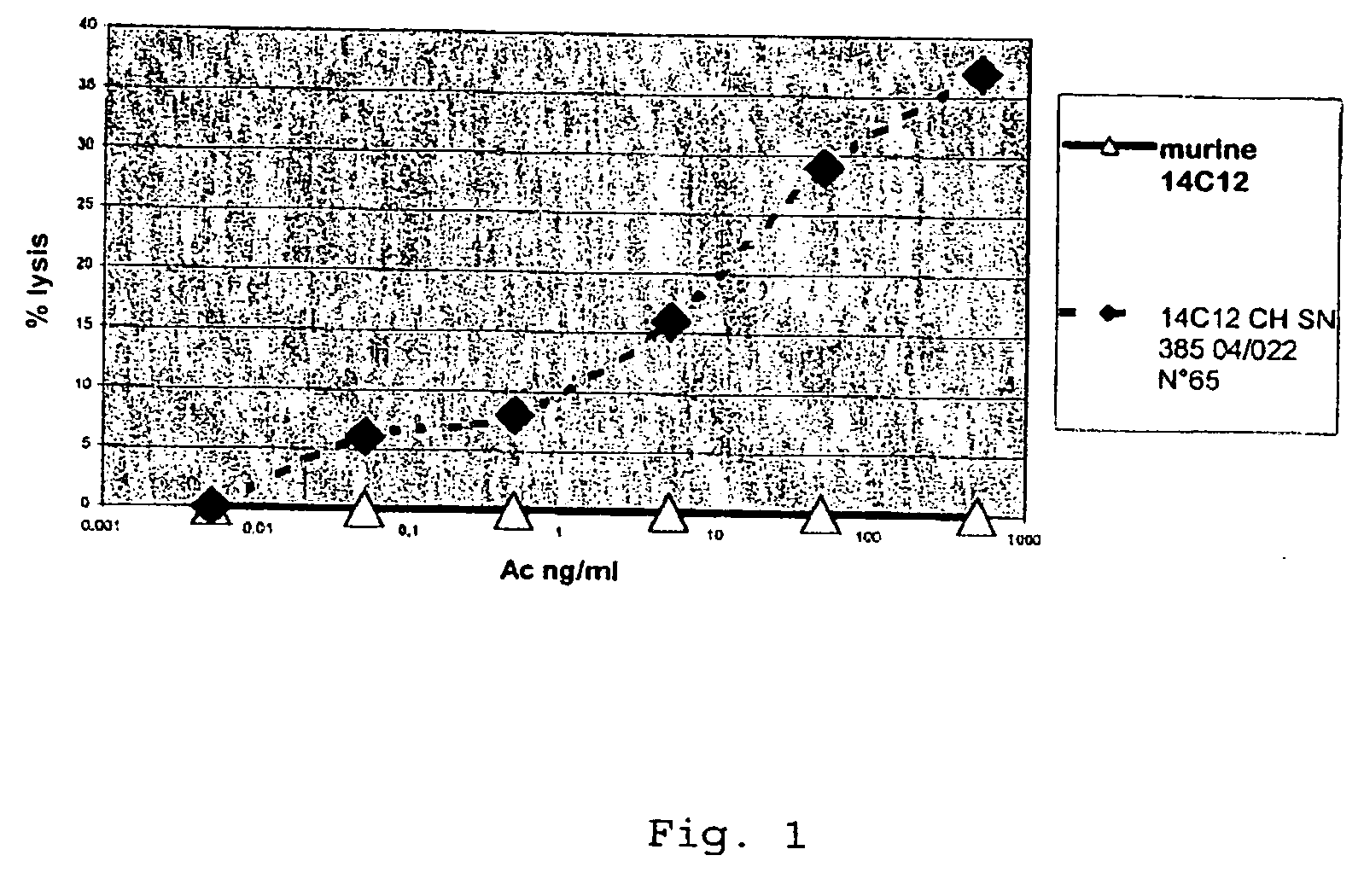
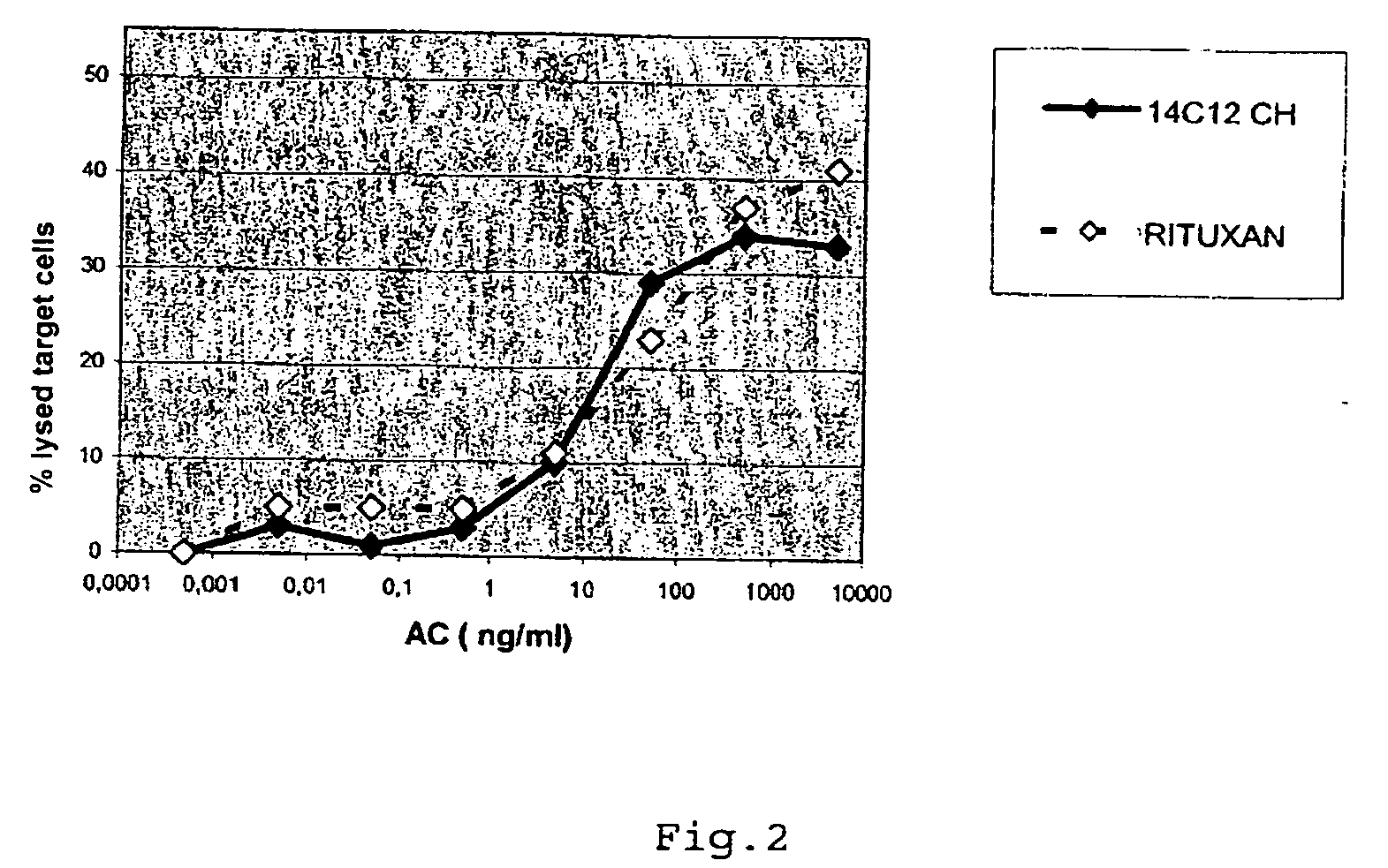
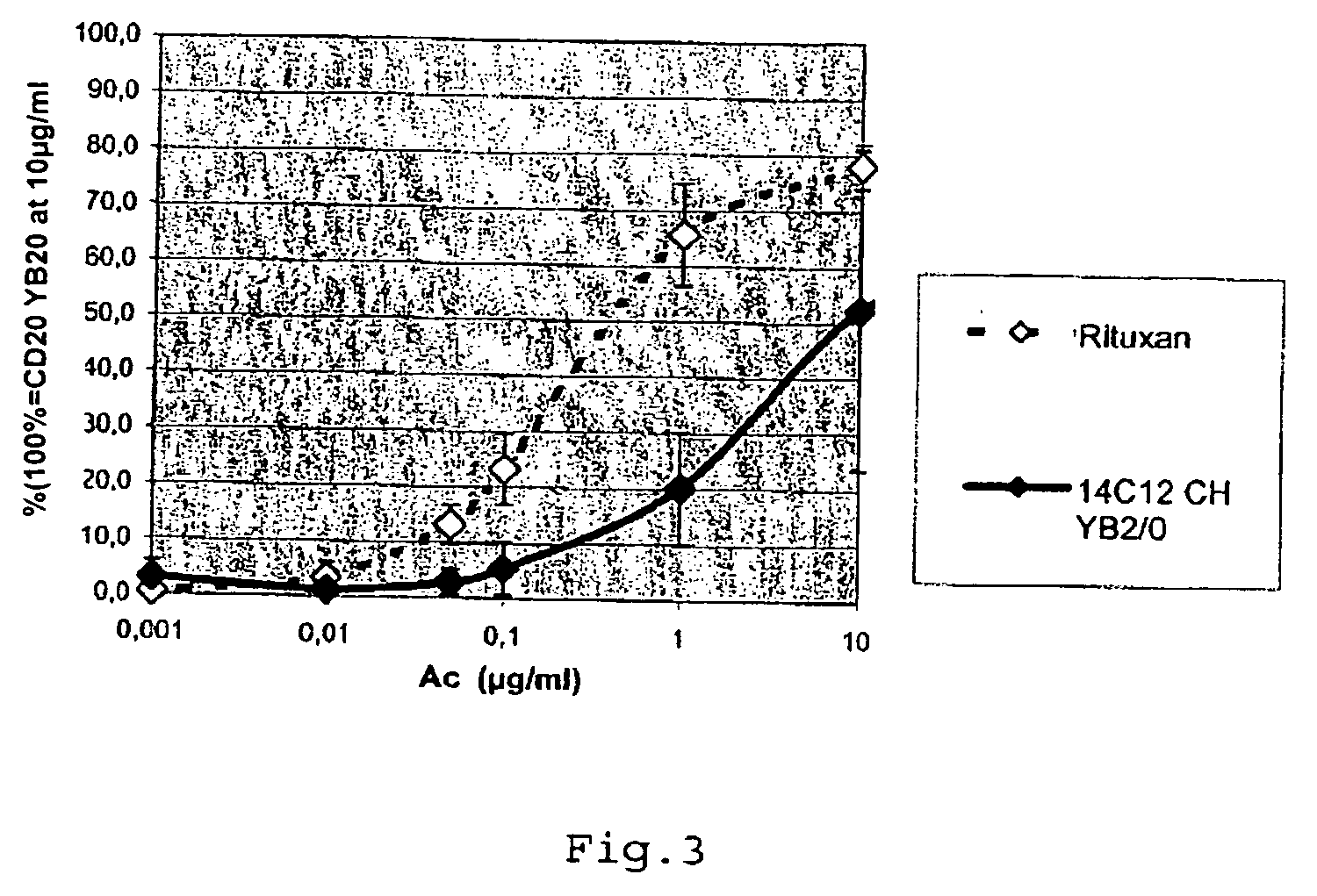
Cytotoxic Antibodies Directed Against Antibodies Inhibiting Factor VIII
InactiveUS20090130094A1Increase the number ofHigh affinityAnimal cellsSugar derivativesCytotoxic antibodyNucleic acid sequencing
The invention relates to an anti-idiotypical antibody targeting an antibody inhibiting the human factor VIII, said inhibiting antibody targeting the C2 region of the human factor VIII, the variable region of each of the light chains thereof being encoded by a sequence of nucleic acids of which at least 70% is identical to the murine sequence of nucleic acids SEQ ID NO: 1, and the variable region of each of the heavy chains thereof being encoded by a sequence of nucleic acids of which at least 70% is identical to the murine sequence of nucleic acids SEQ ID NO: 2, the constant regions of the light chains and the heavy chains being constant regions from a non-murine species. The invention also relates to the use of said antibody for activating the FcγRIII receptors of cytotoxic immune cells, and to the production of a medicament especially for the treatment of haemophilia A.
Owner:LFB BIOTECH
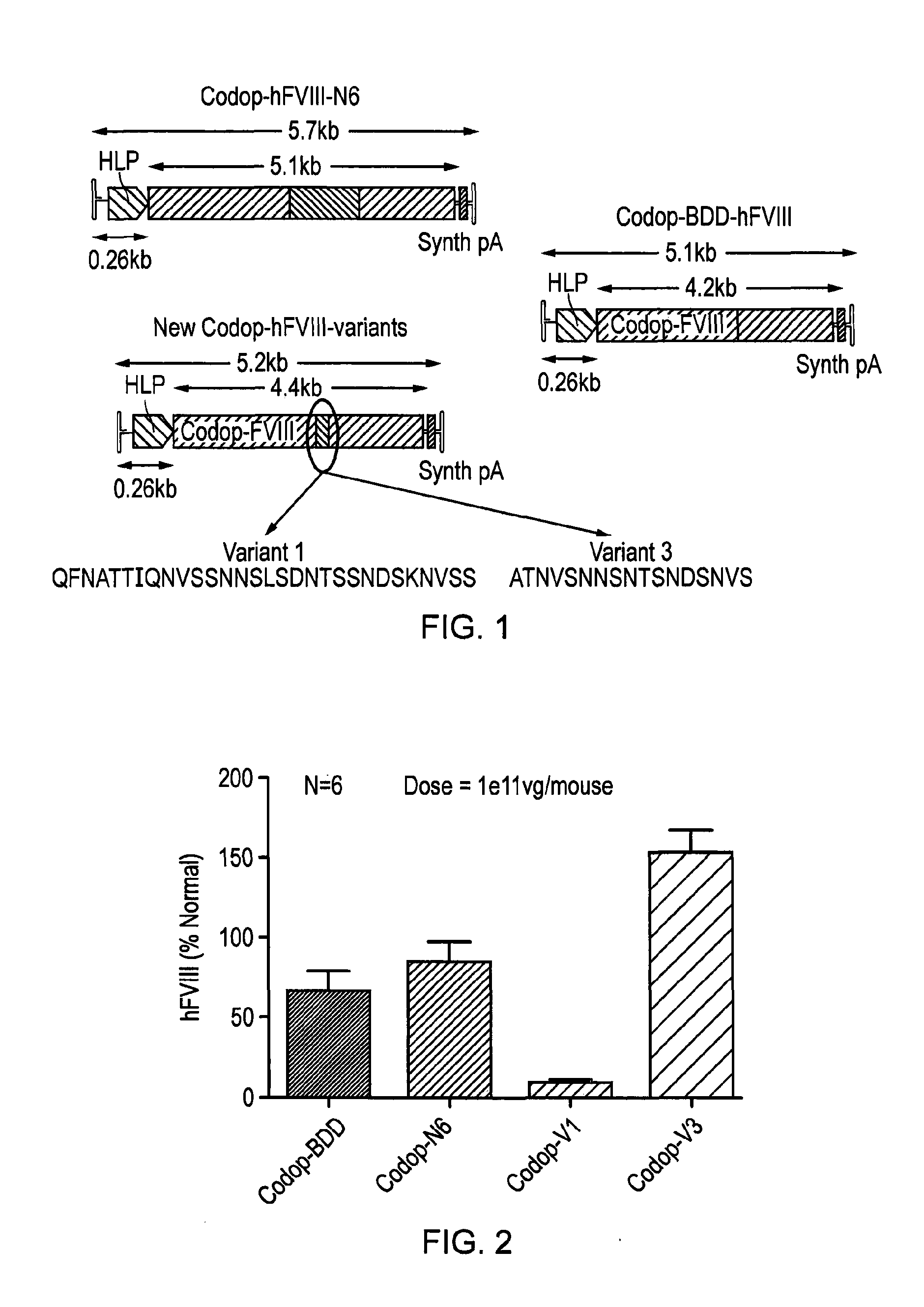
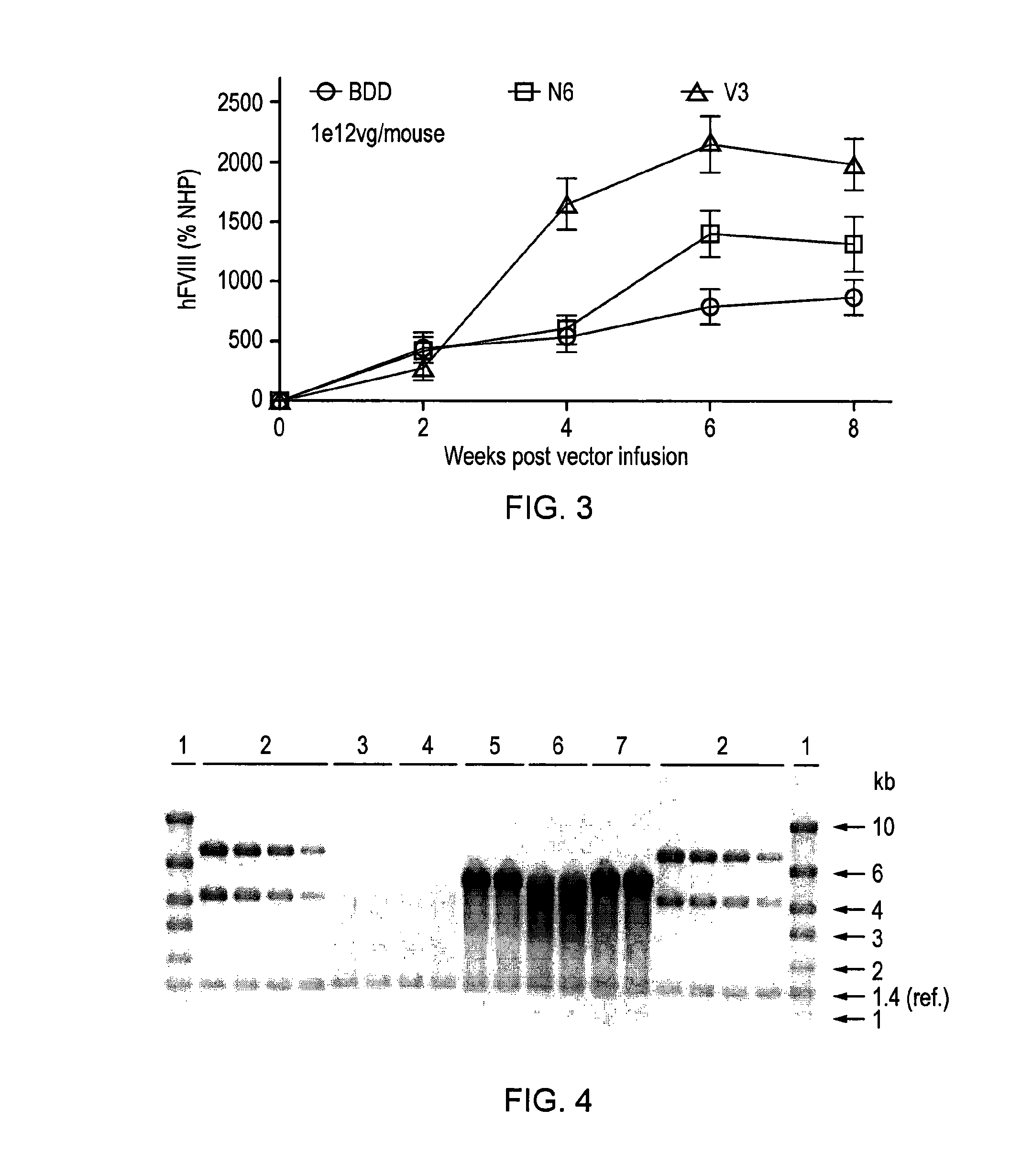
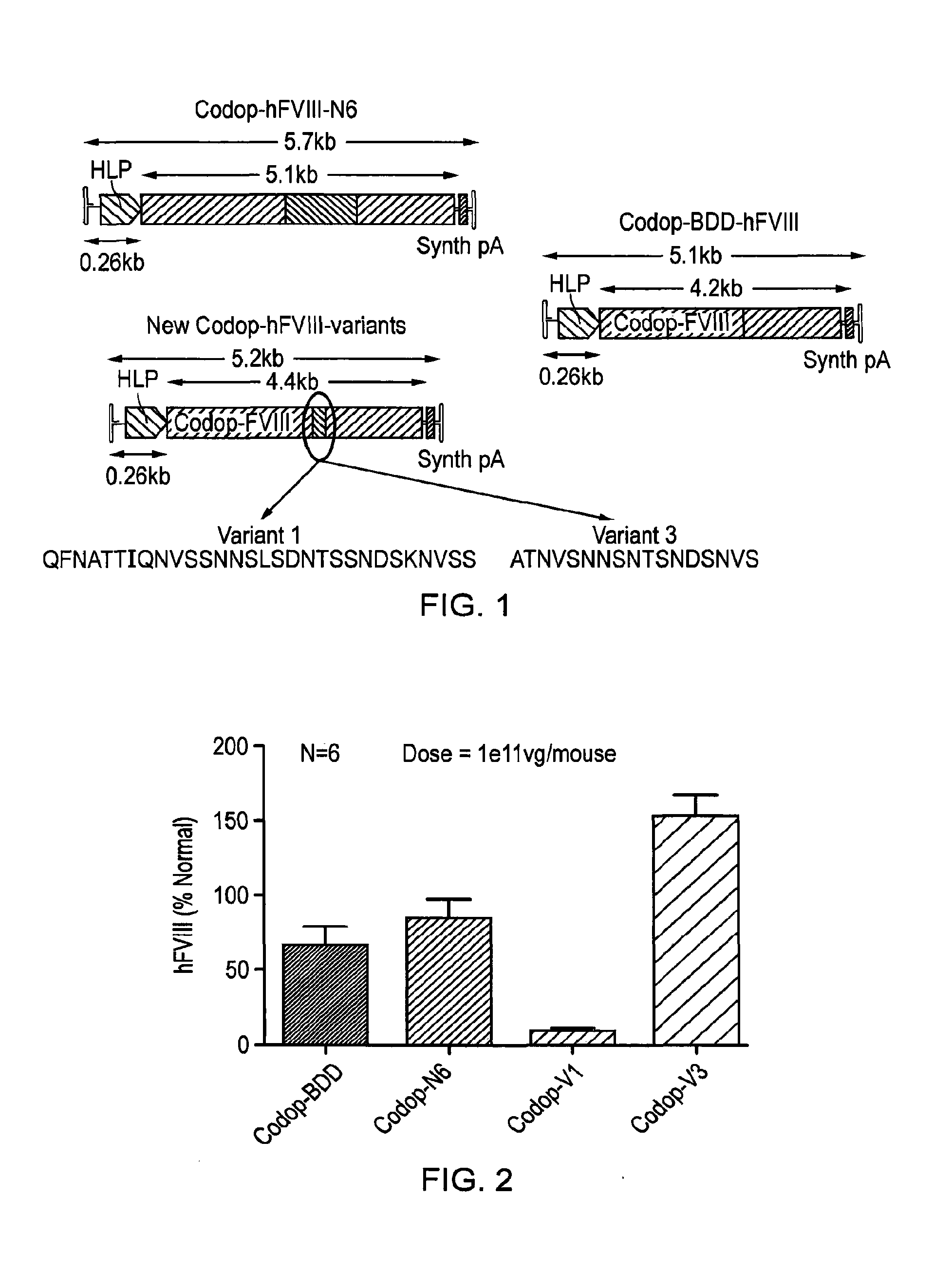
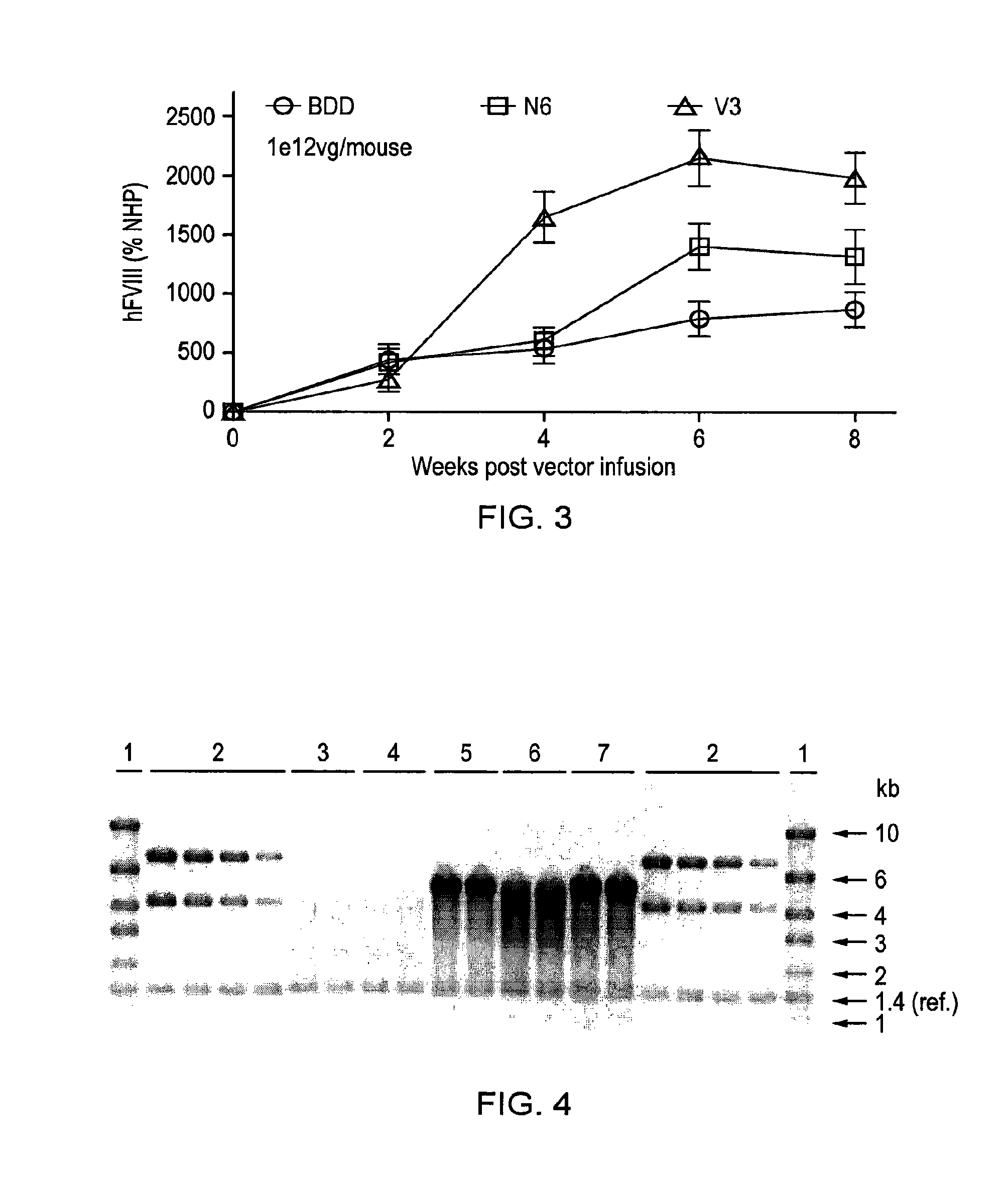
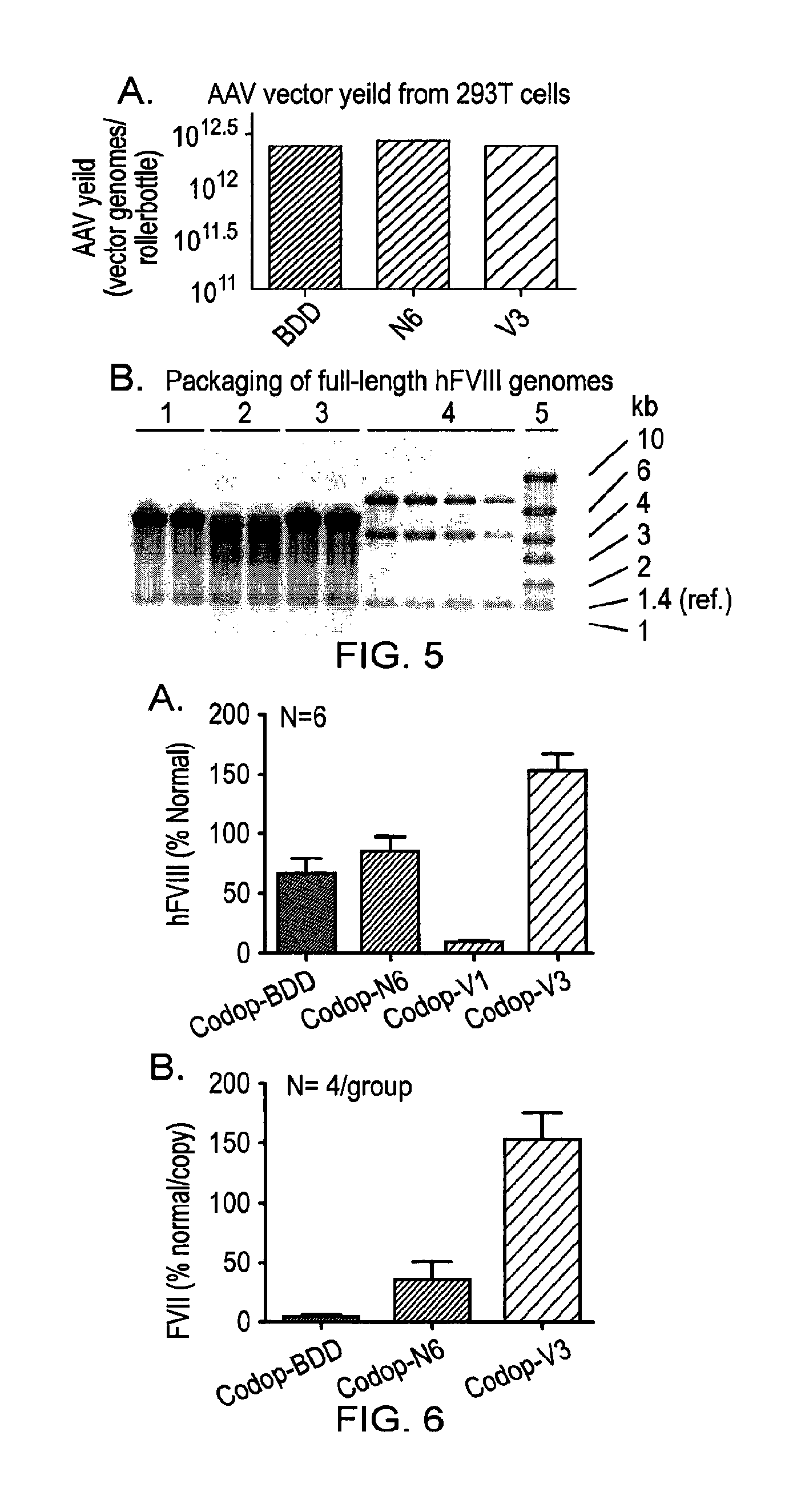
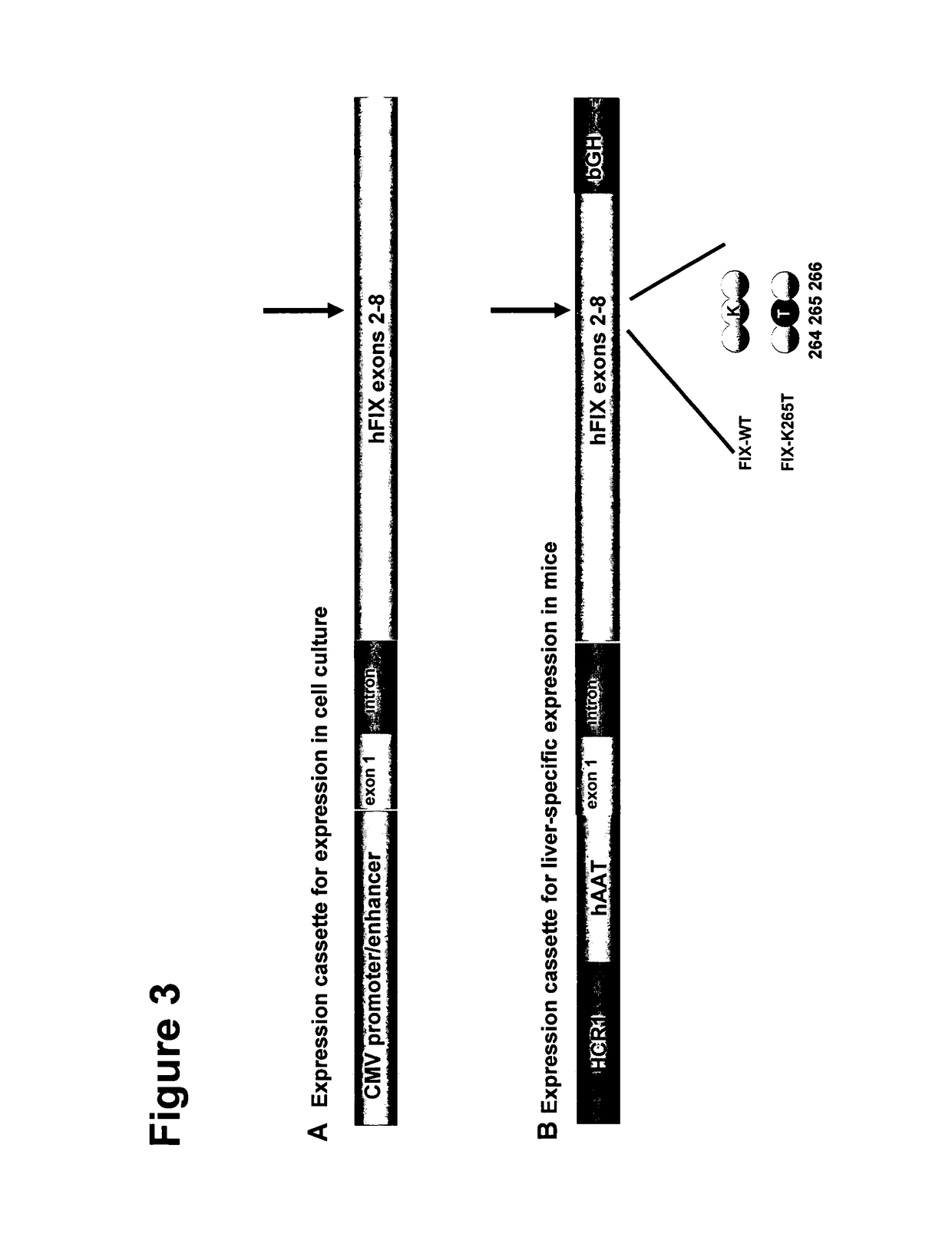
Vectors for liver-directed gene therapy of hemophilia and methods and use thereof
ActiveUS10398787B2Improve safety and efficiencyIncrease gene expressionVectorsPeptide/protein ingredientsGeneRegulatory sequence
The present invention relates to vectors containing liver-specific regulatory sequences and codon-optimized factor IX or factor VIII genes, methods employing these vectors and uses of these vectors. Expression cassettes and vectors containing these liver-specific regulatory elements and codon-optimized factor IX or factor VIII genes are also disclosed. The present invention is particularly useful for applications using gene therapy, in particular for the treatment of hemophilia A and B.
Owner:VRIJE UNIV BRUSSEL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






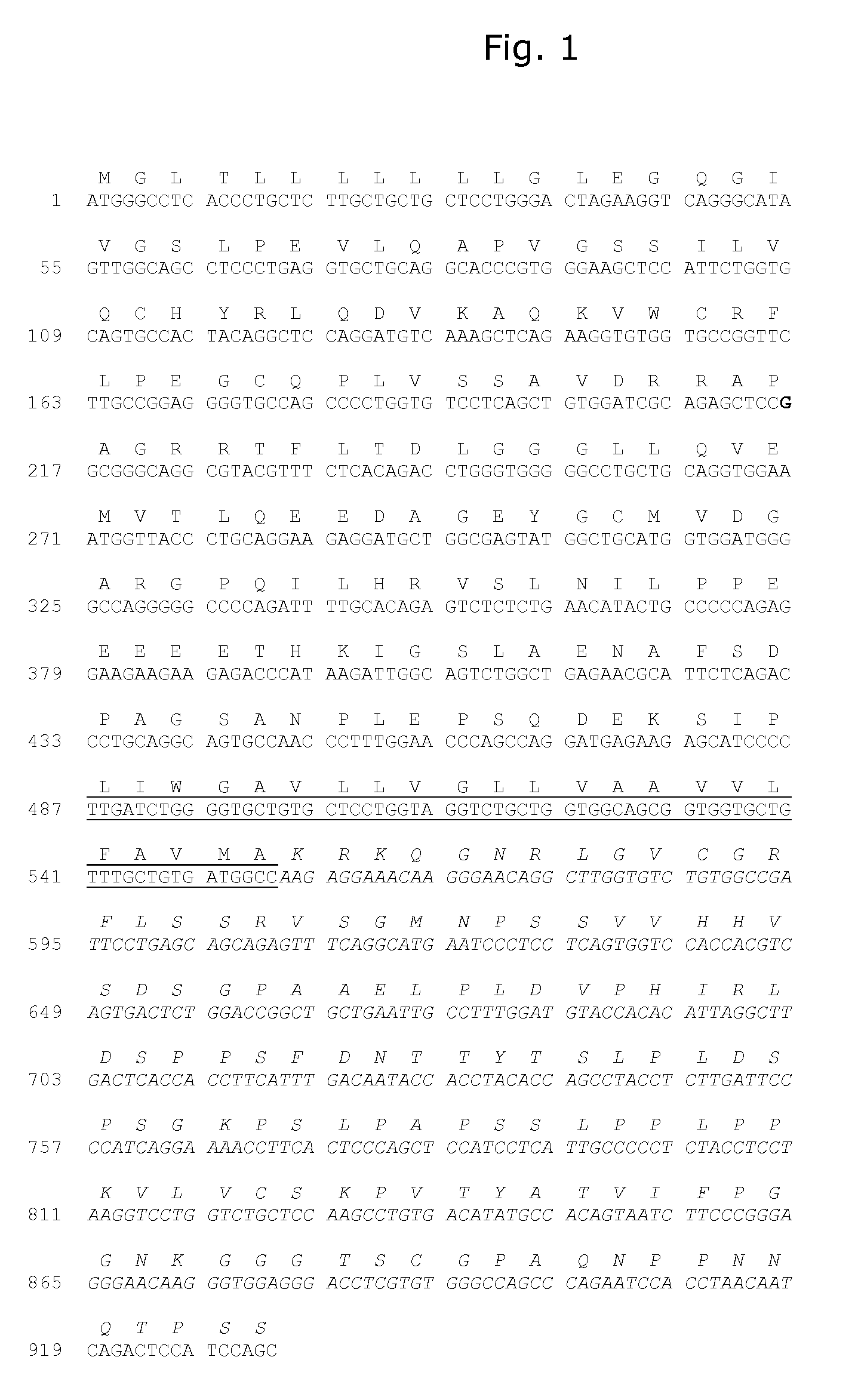


![Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a](https://images-eureka.patsnap.com/patent_img/de8b7244-99c8-43b2-81e4-d70e4ac6493c/US20100197578A1-20100805-D00001.png)
![Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a](https://images-eureka.patsnap.com/patent_img/de8b7244-99c8-43b2-81e4-d70e4ac6493c/US20100197578A1-20100805-D00002.png)
![Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a Demannosylated recombinant factor viii [[vii]] for the treatment of patients with haemophilia a](https://images-eureka.patsnap.com/patent_img/de8b7244-99c8-43b2-81e4-d70e4ac6493c/US20100197578A1-20100805-D00003.png)