Patents
Literature
127 results about "Protein activation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The protein C zymogen is activated when it binds to thrombin, another protein heavily involved in coagulation, and protein C's activation is greatly promoted by the presence of thrombomodulin and endothelial protein C receptors (EPCRs).
Assay for rapid detection of human activated protein C and highly specific monoclonal antibody therefor
InactiveUS6989241B2High selectivityStrong specificityAnimal cellsMicrobiological testing/measurementProtein activationHybridoma cell
Owner:OKLAHOMA MEDICAL RES FOUND
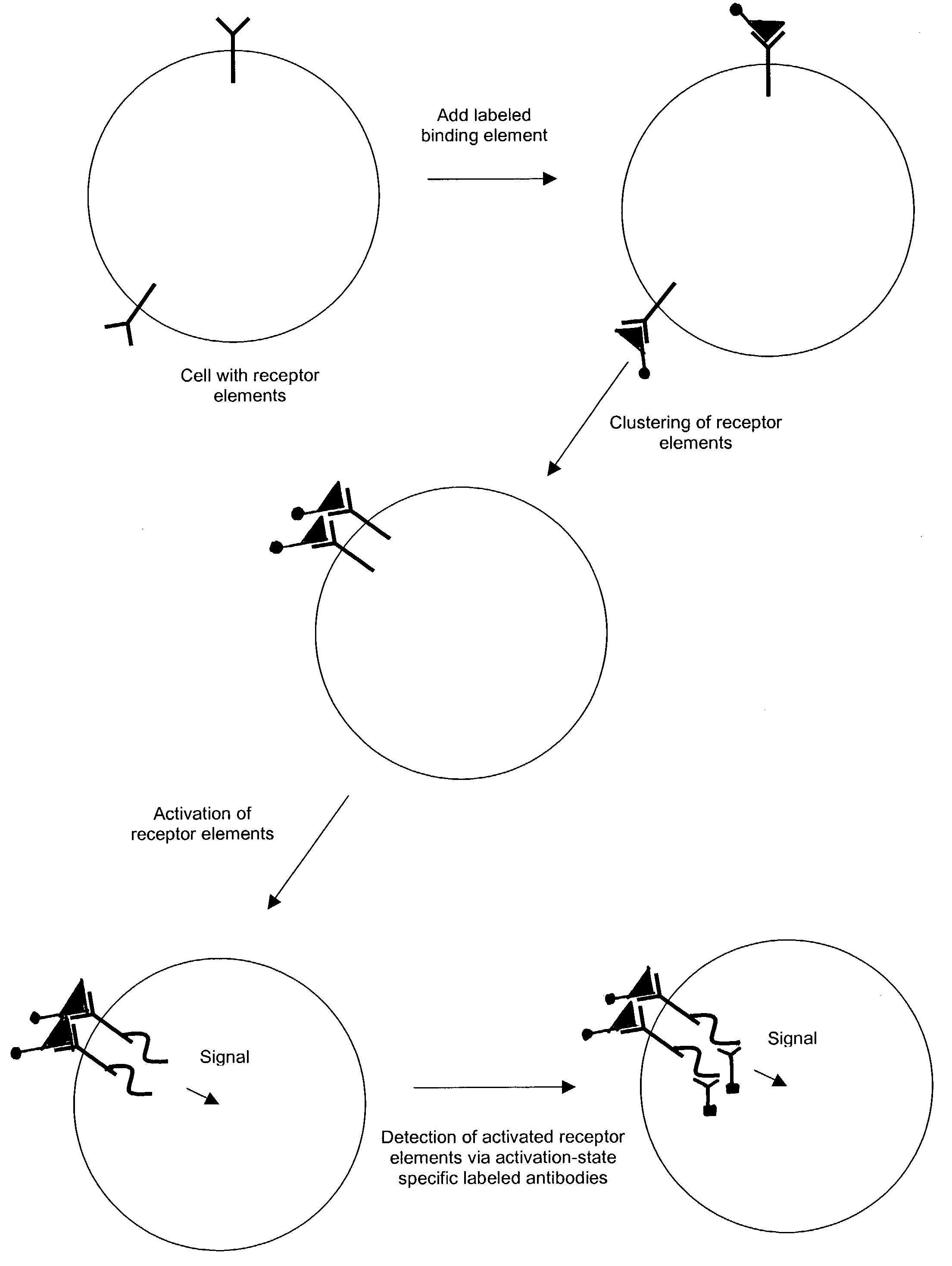
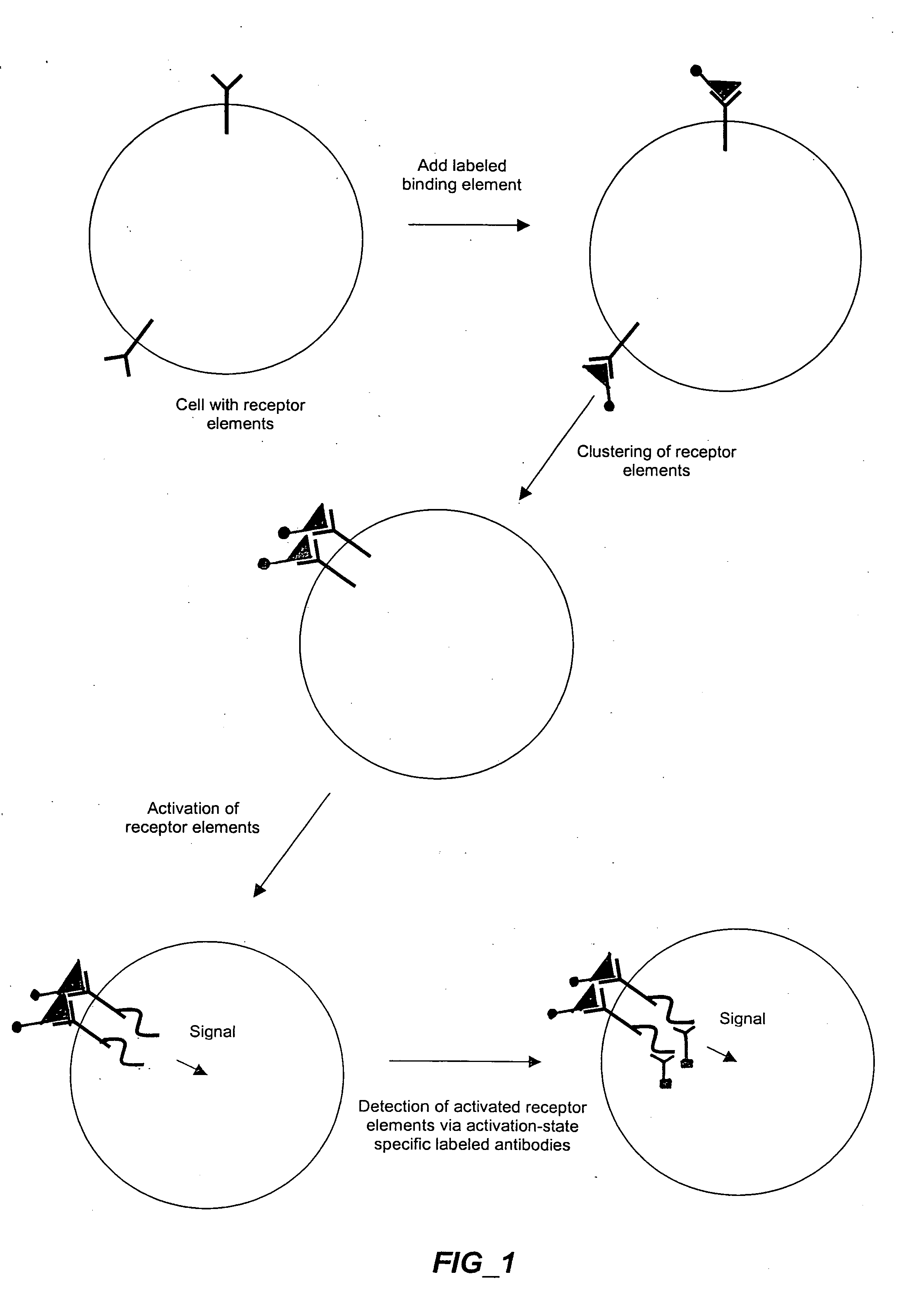
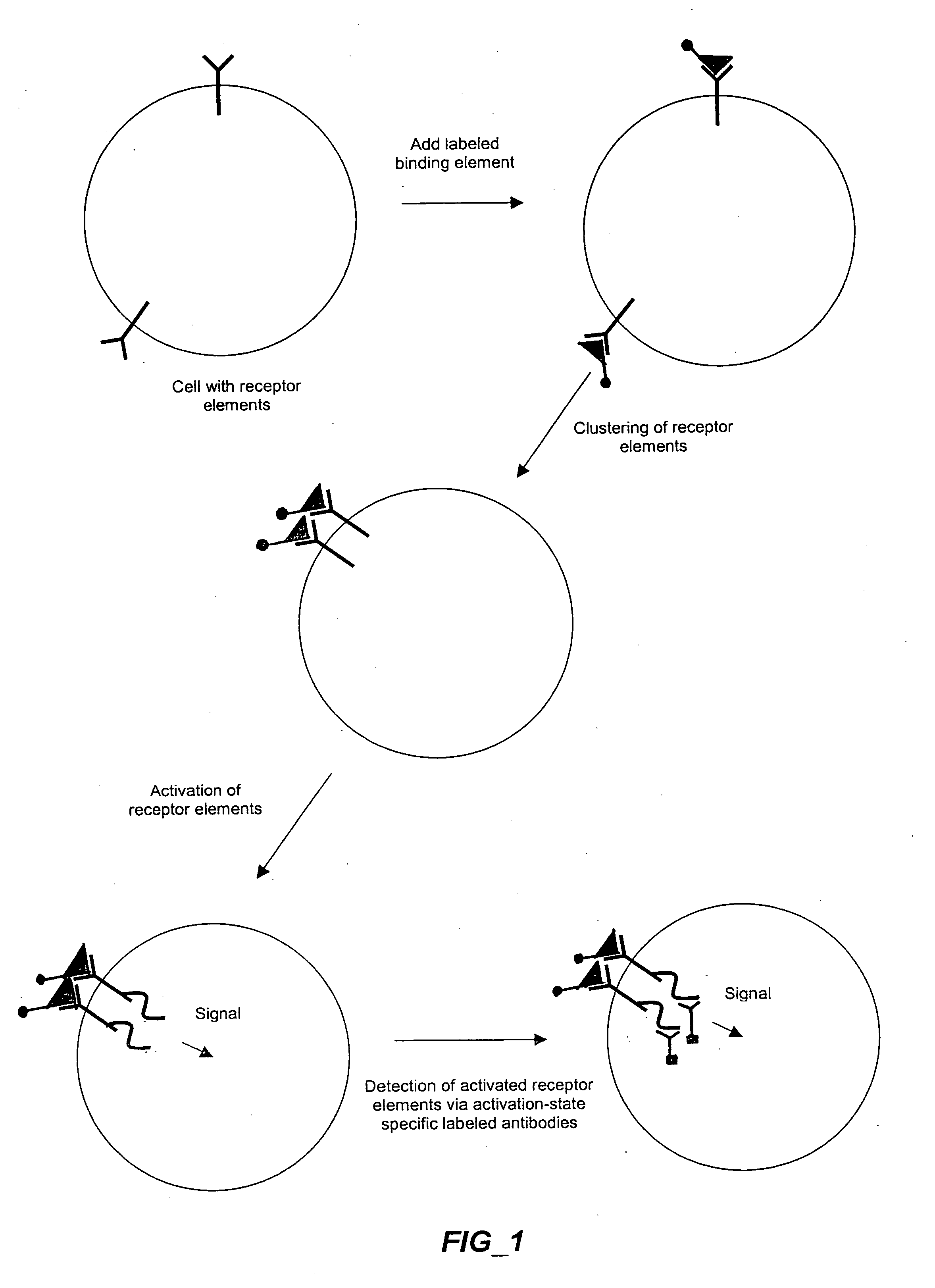
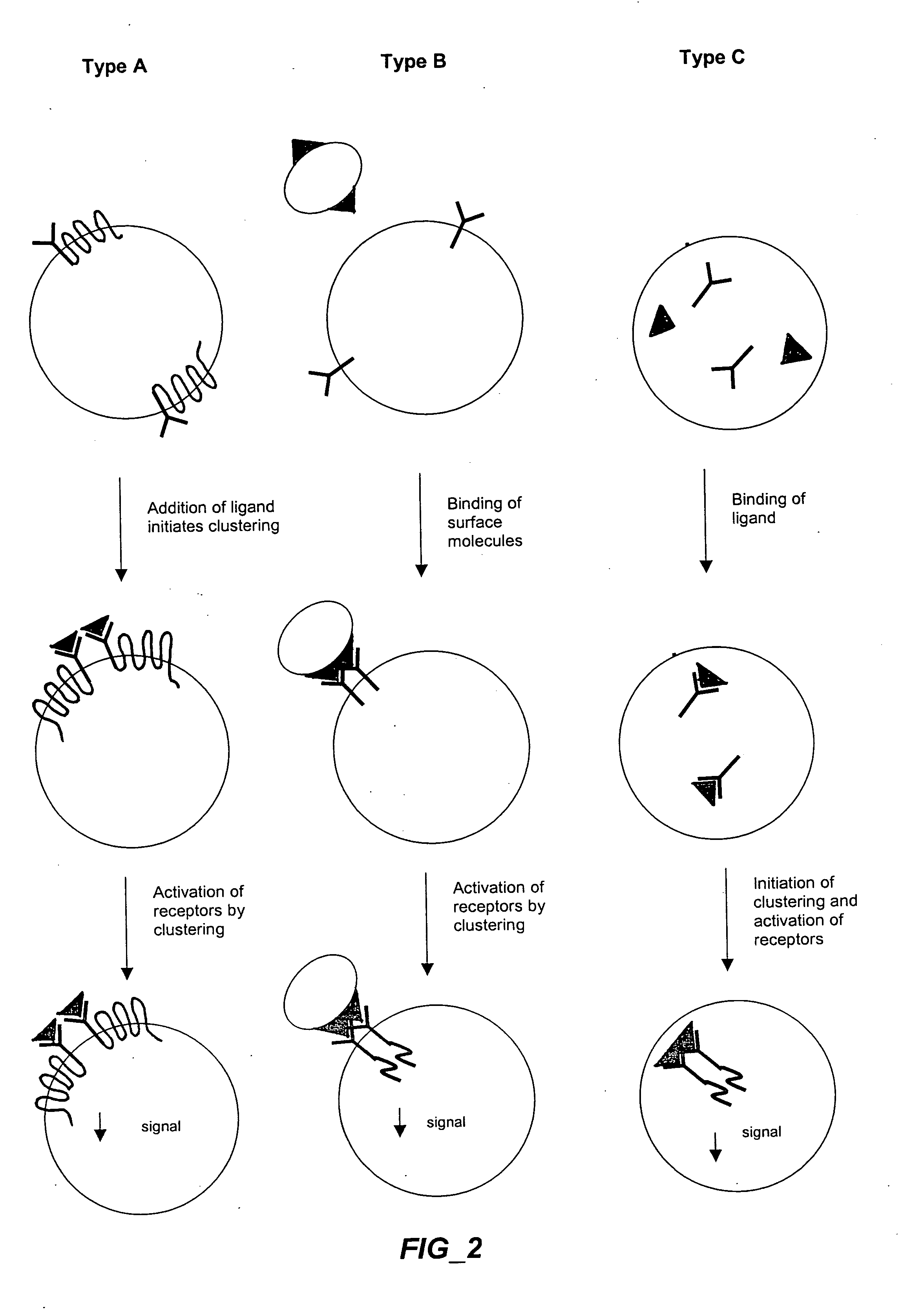
Methods and compositions for detecting receptor-ligand interactions in single cells
InactiveUS7381535B2Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseProtein activation
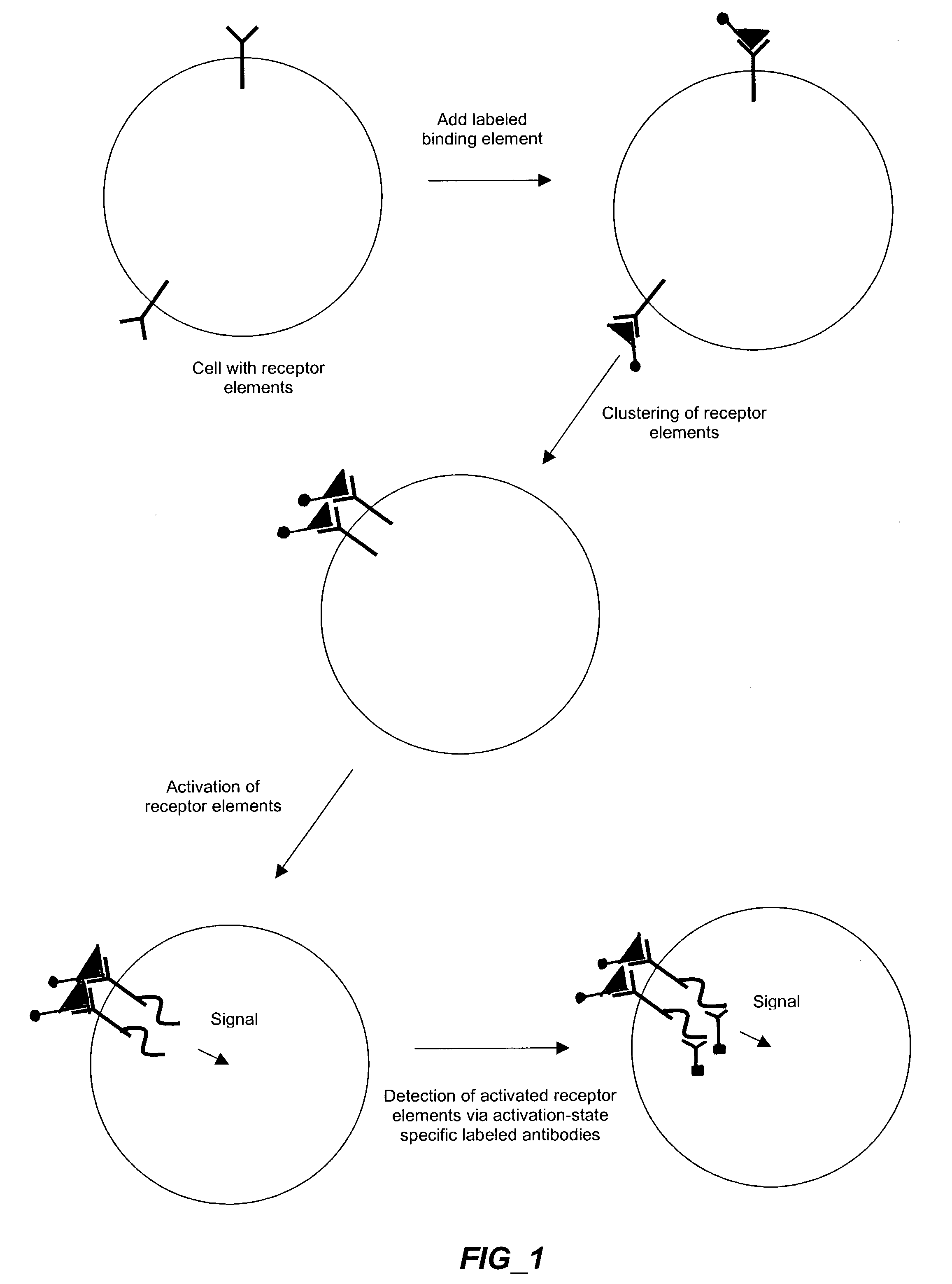
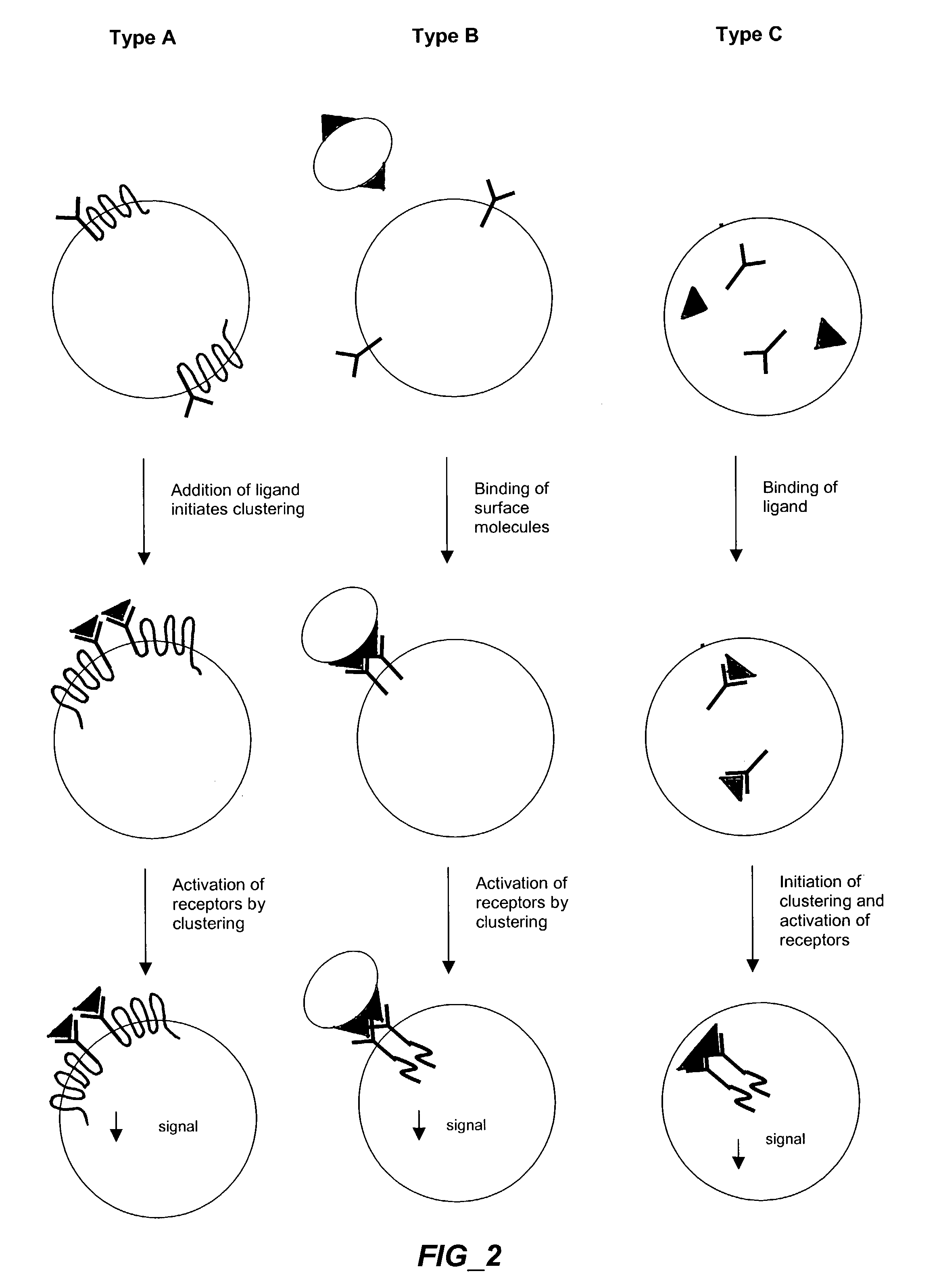
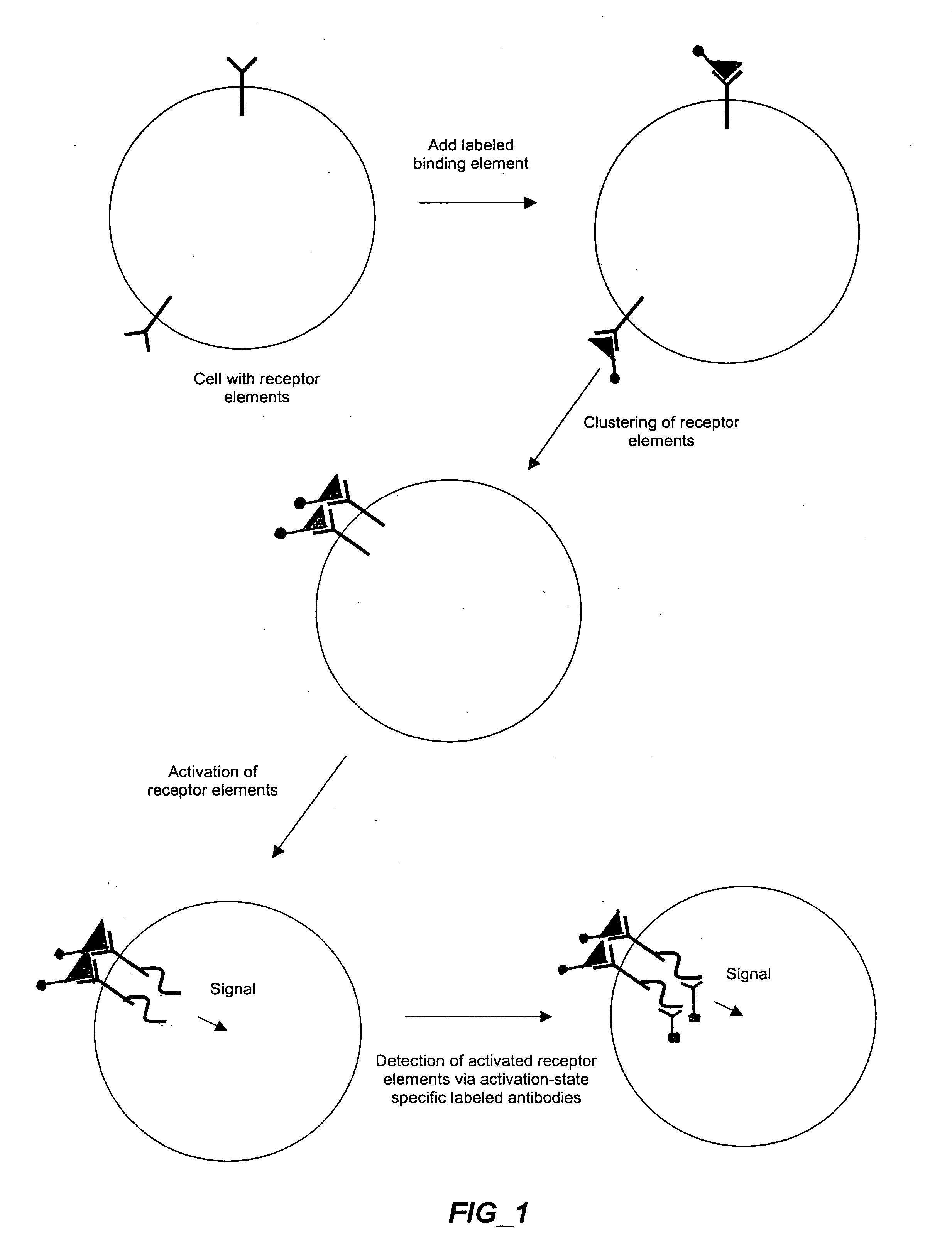
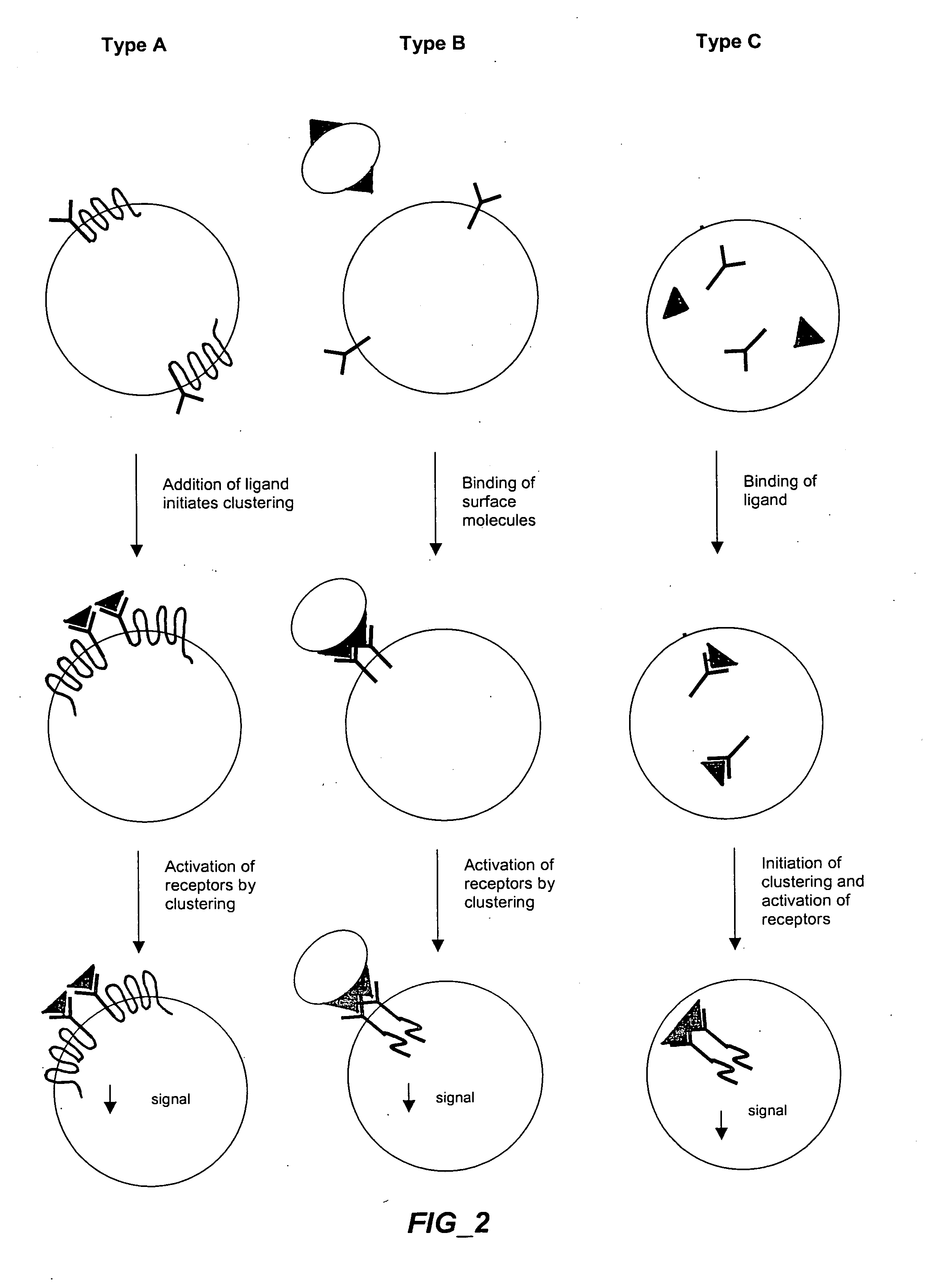
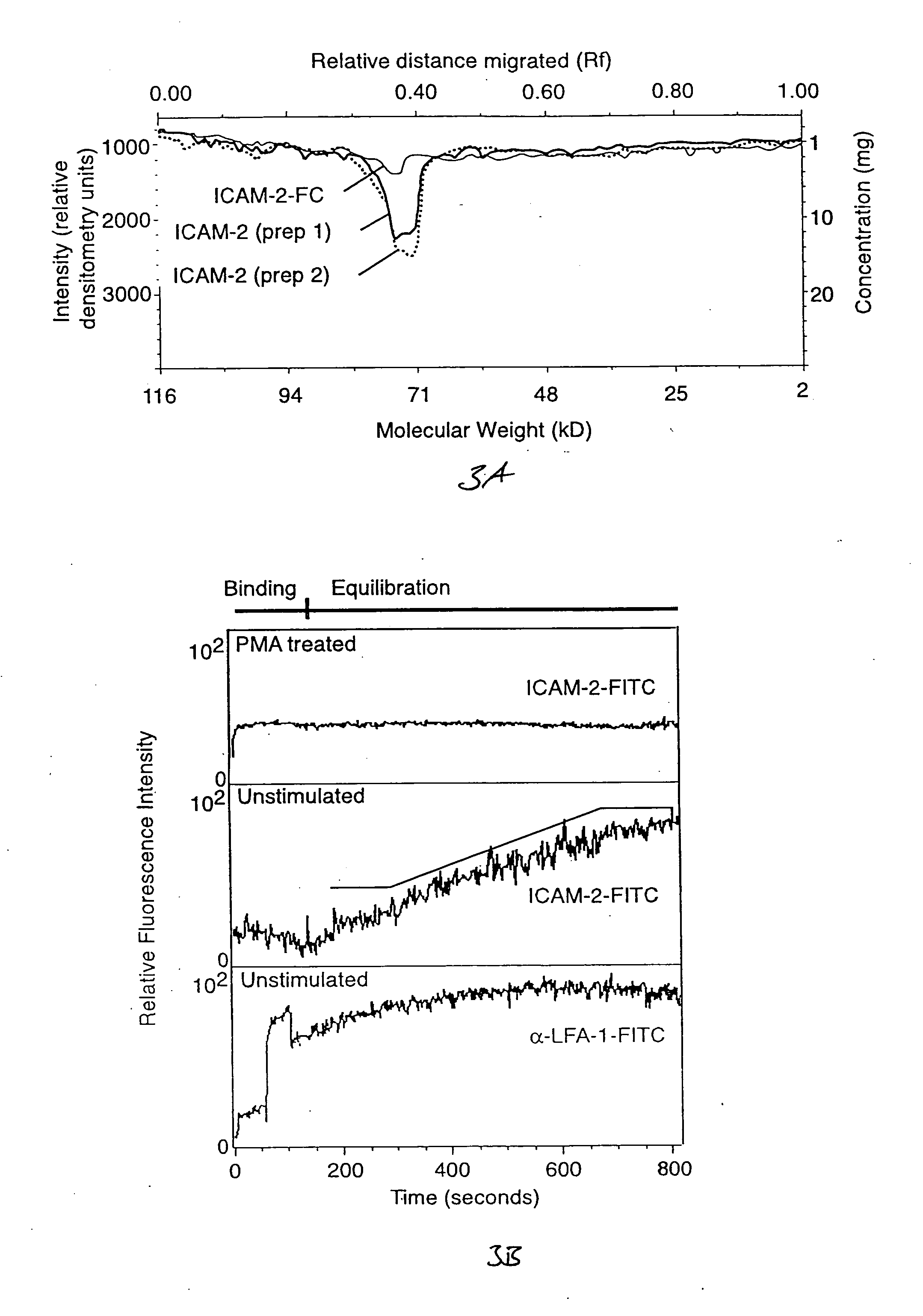
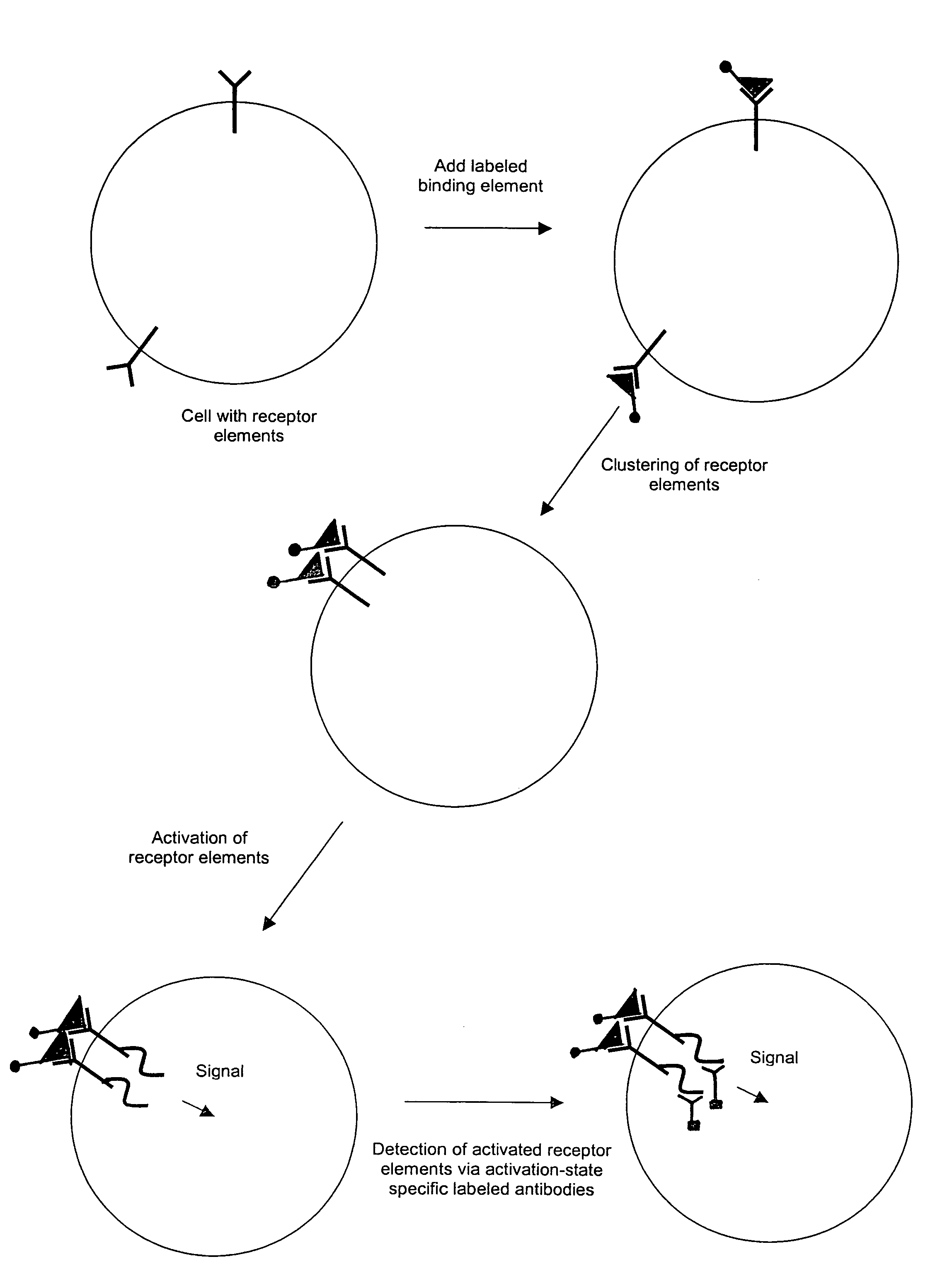
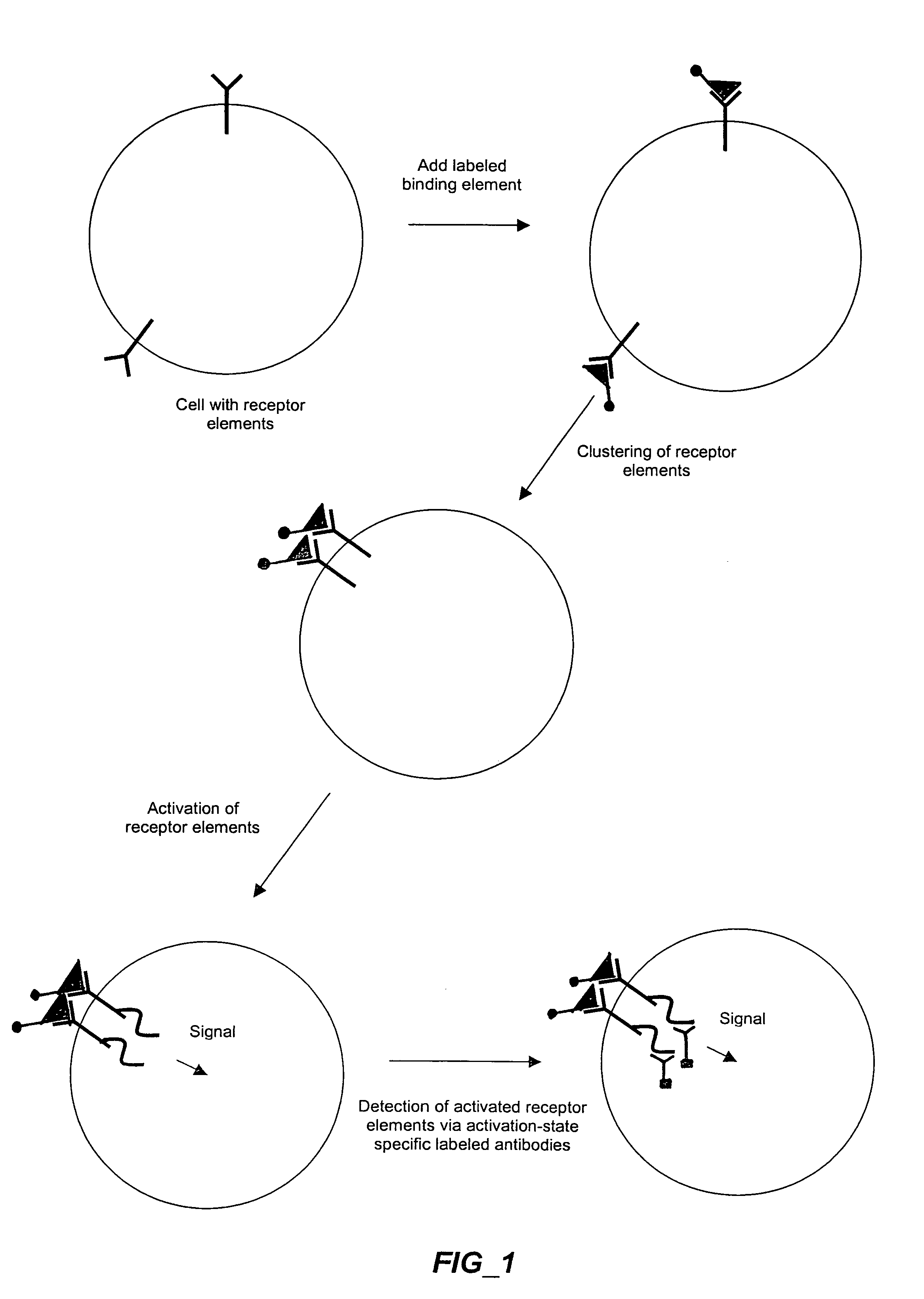
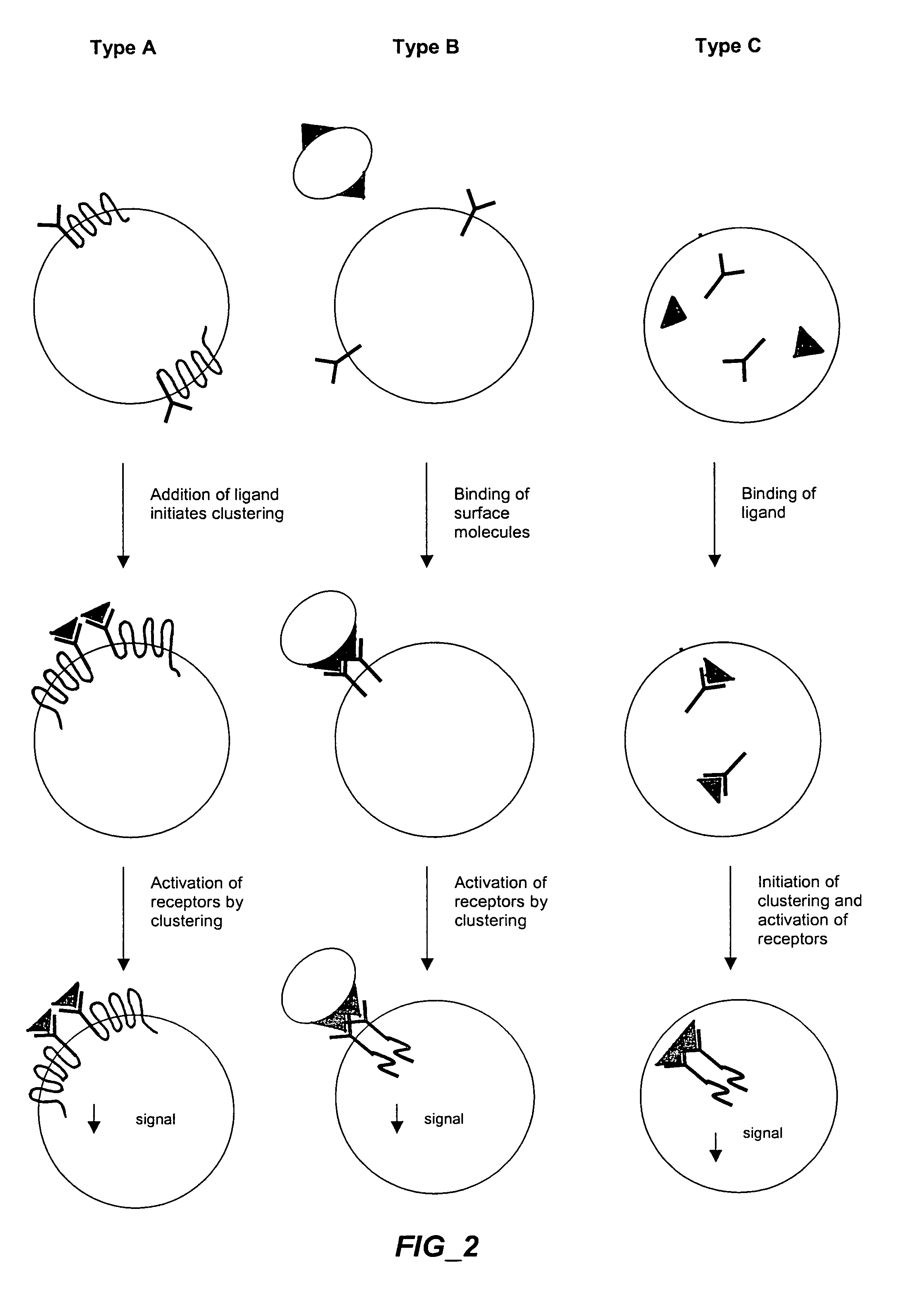
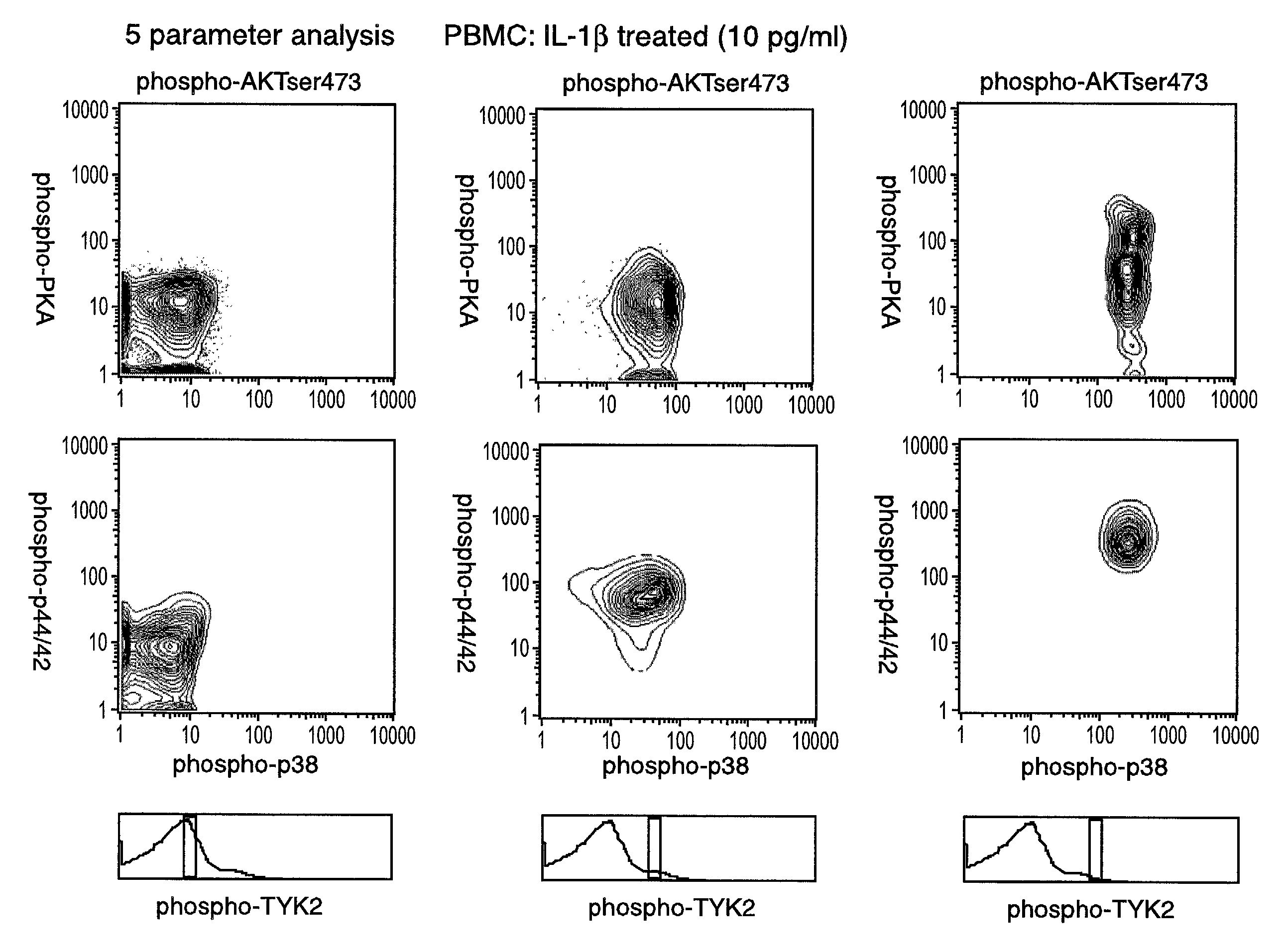
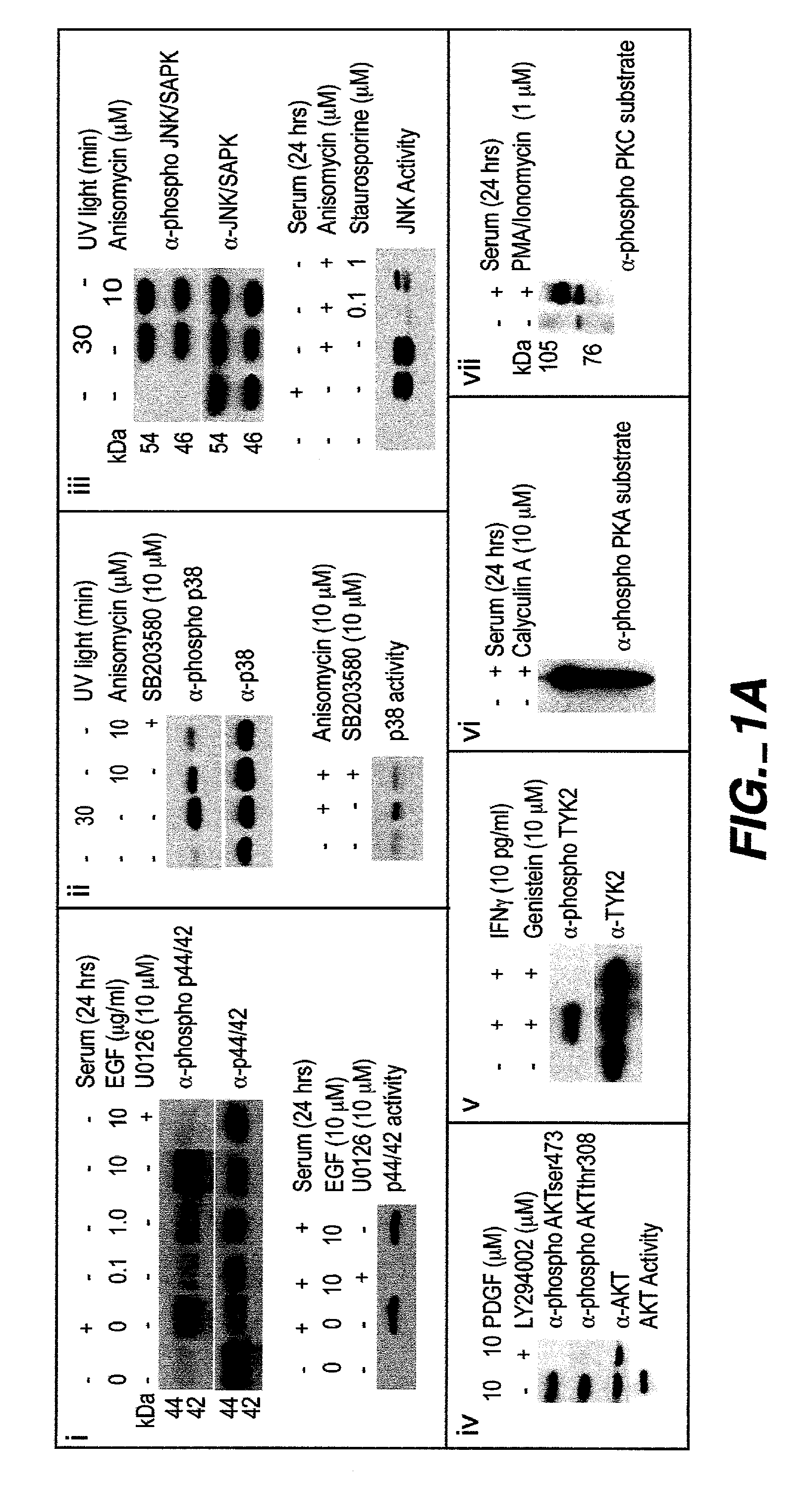
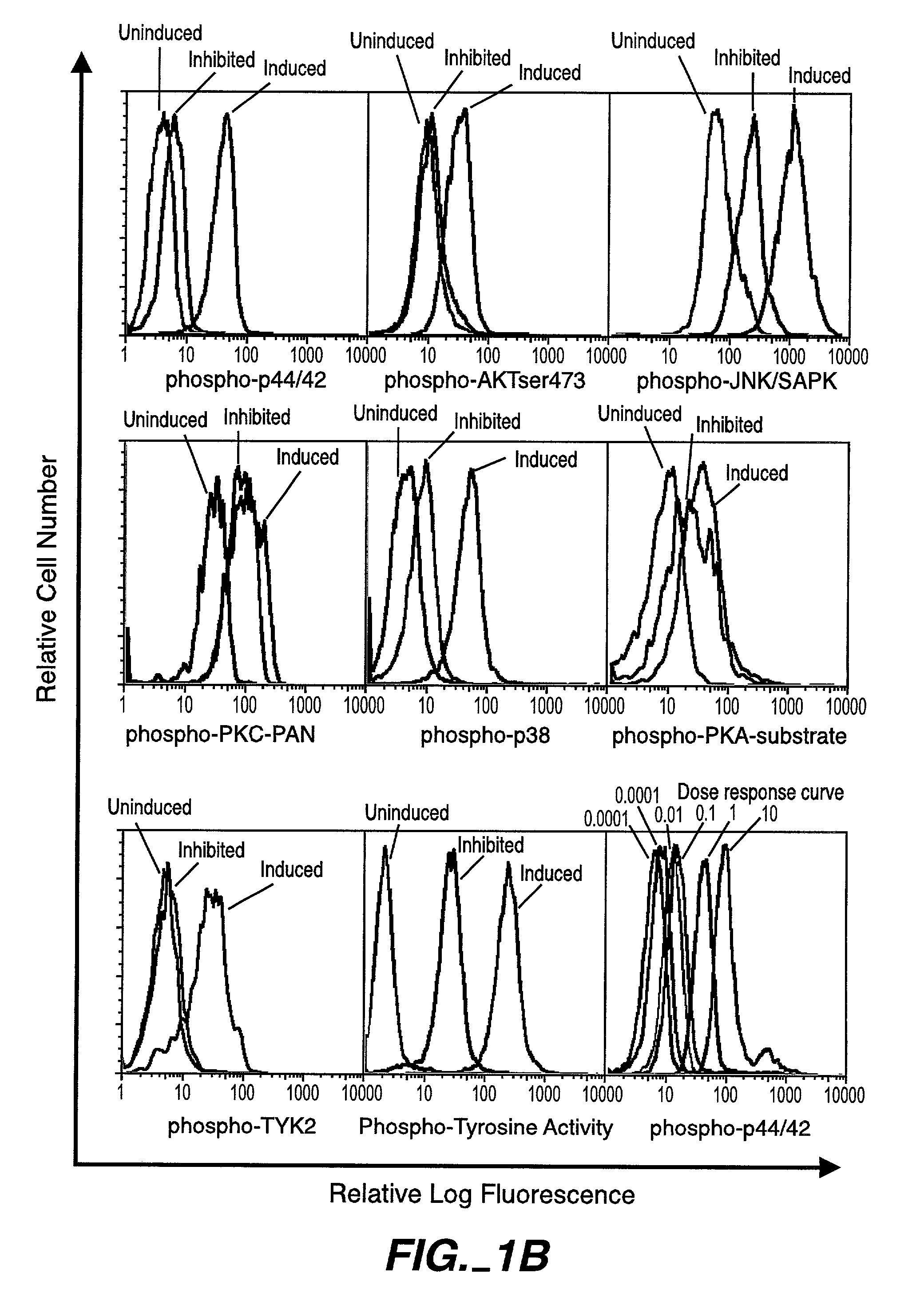
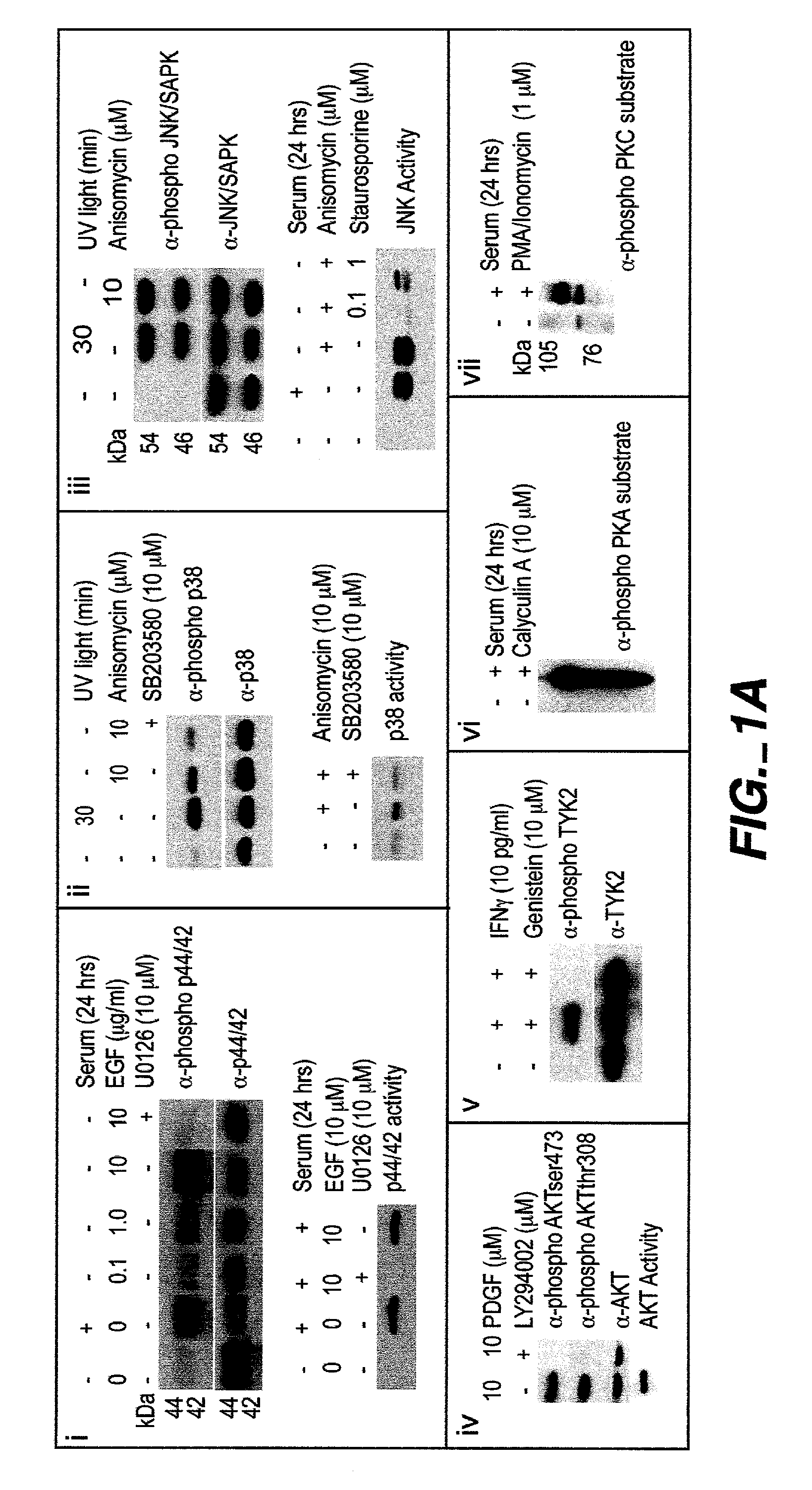
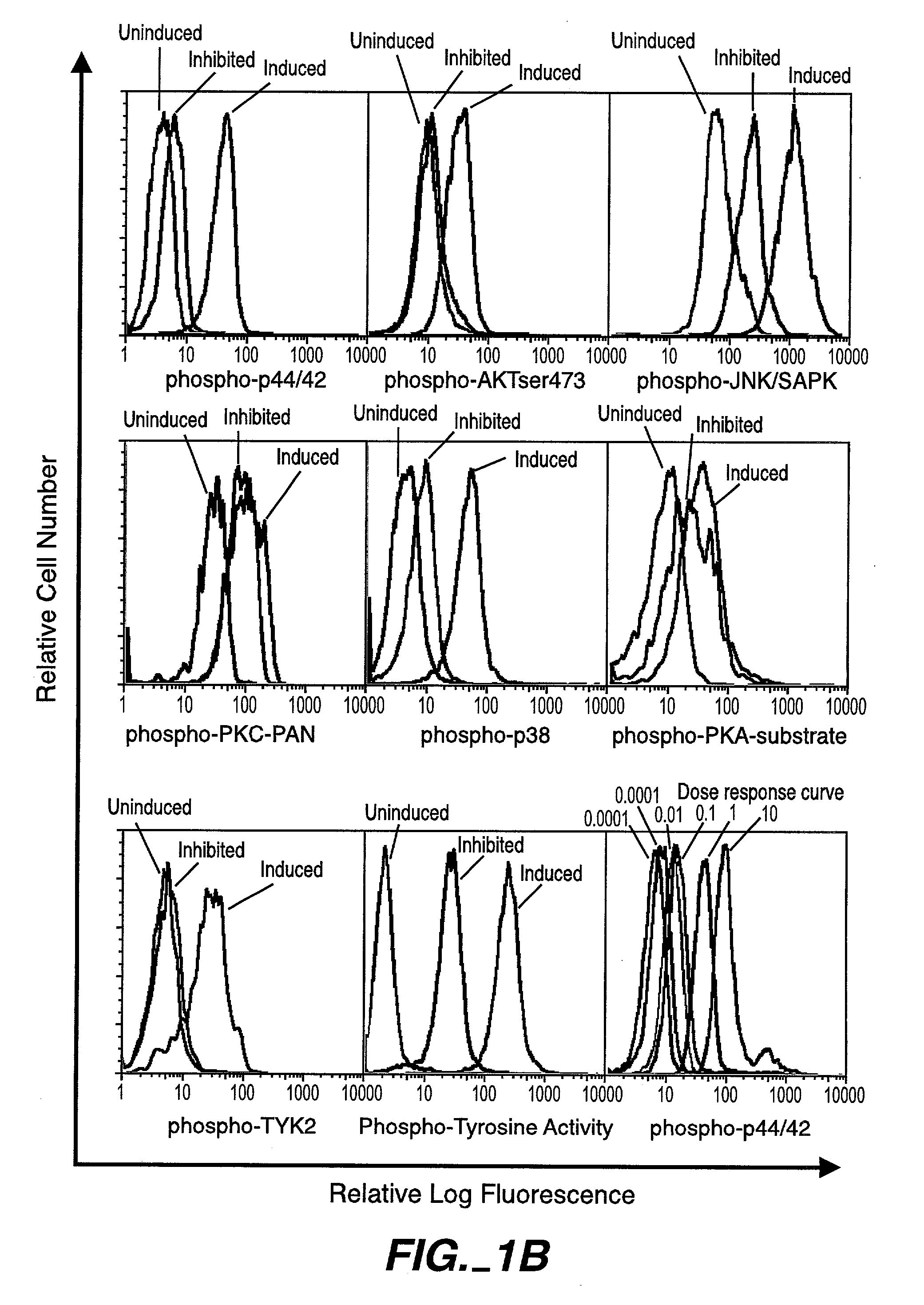
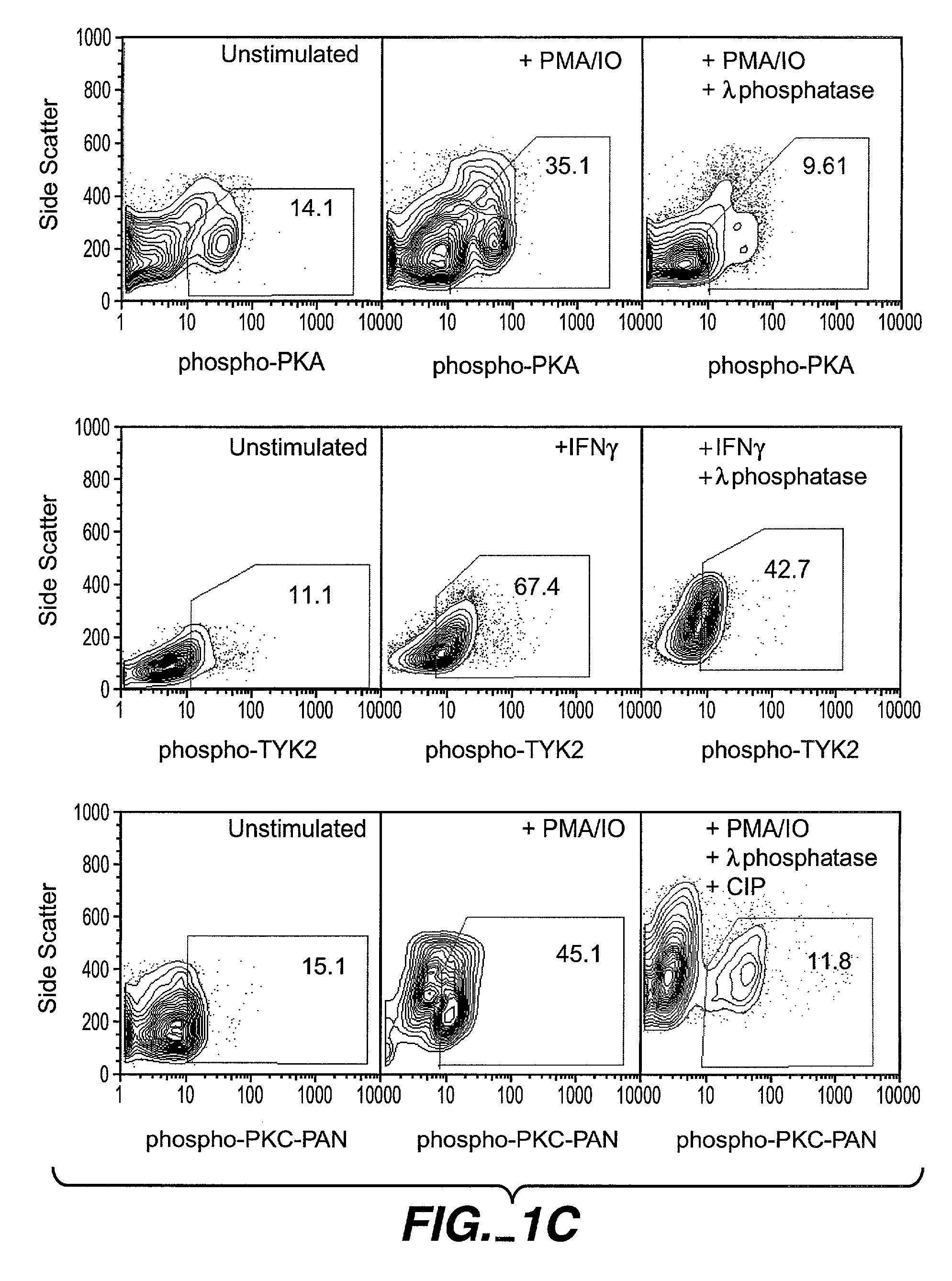
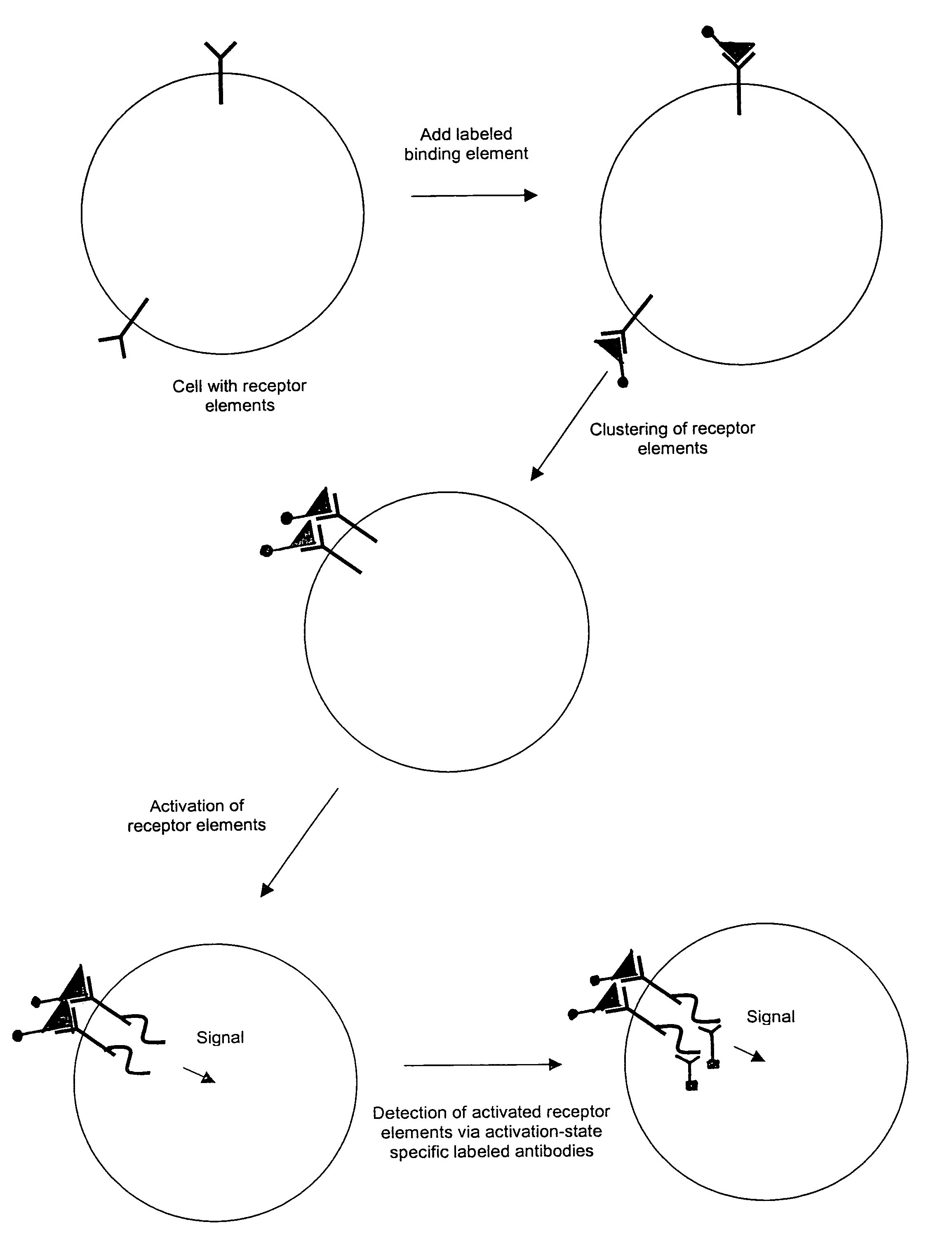
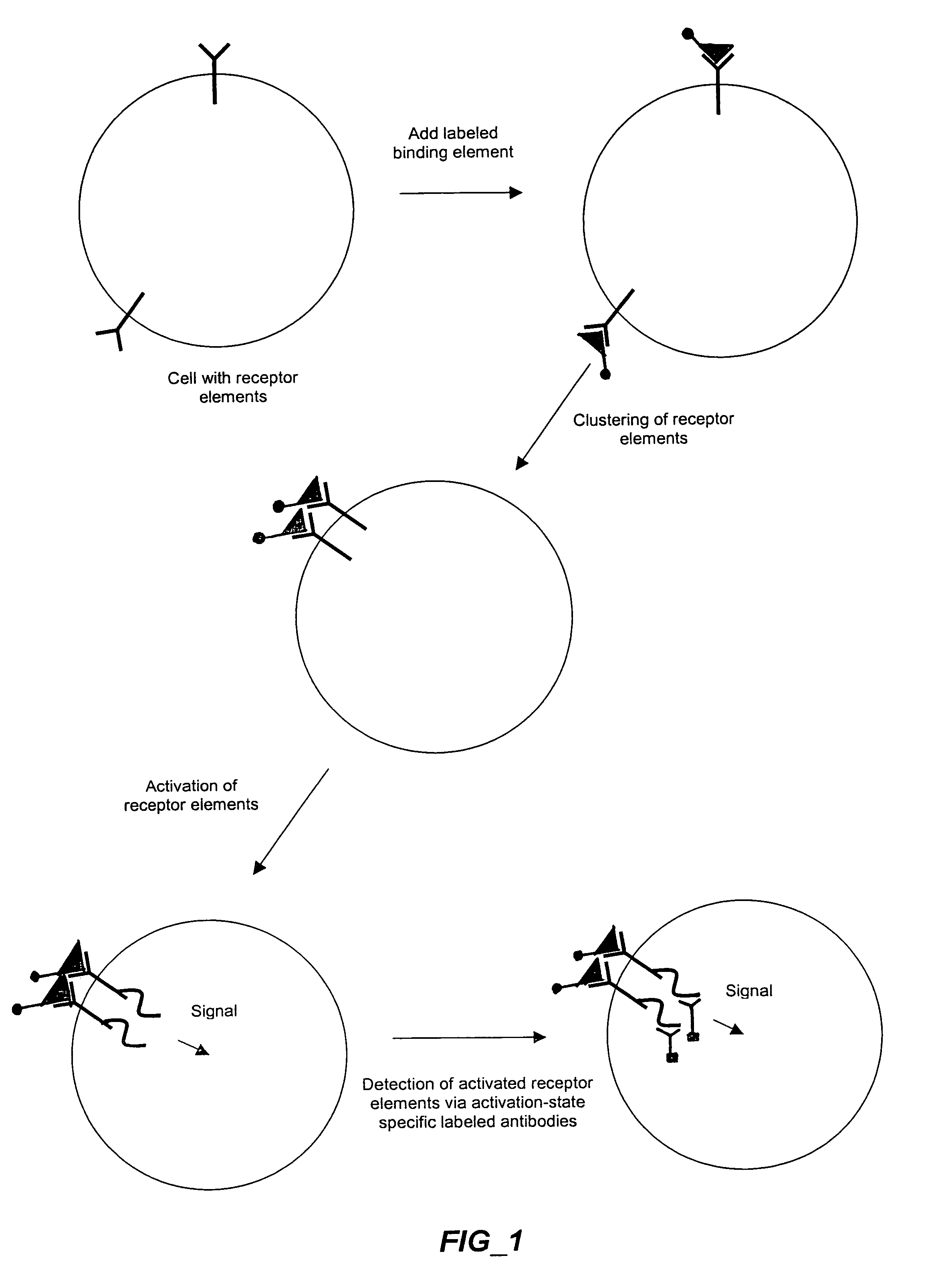
The invention provides methods and compositions for simultaneously detecting the activation state of a plurality of proteins in single cells using flow cytometry. The invention further provides methods and compositions of screening for bioactive agents capable of coordinately modulating the activity of a plurality of proteins in single cells. The methods and compositions can be used to determine the protein activation profile of a cell for predicting or diagnosing a disease state, and for monitoring treatment of a disease state.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for detecting receptor-ligand interactions in single cells
The invention provides methods and compositions for simultaneously detecting the activation state of a plurality of proteins in single cells using flow cytometry. The invention further provides methods and compositions of screening for bioactive agents capable of coordinately modulating the activity of a plurality of proteins in single cells. The methods and compositions can be used to determine the protein activation profile of a cell for predicting or diagnosing a disease state, and for monitoring treatment of a disease state.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for detecting receptor-ligand interactions in single cells
InactiveUS7695926B2Microbiological testing/measurementPreparing sample for investigationProtein activationBiological activation
The invention provides methods and compositions for simultaneously detecting the activation state of a plurality of proteins in single cells using flow cytometry. The invention further provides methods and compositions of screening for bioactive agents capable of coordinately modulating the activity of a plurality of proteins in single cells. The methods and compositions can be used to determine the protein activation profile of a cell for predicting or diagnosing a disease state, and for monitoring treatment of a disease state.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for detecting the activation state of multiple proteins in single cells
InactiveUS7563584B2Bioreactor/fermenter combinationsBiological substance pretreatmentsProtein activationBiological activation
The invention provides methods and compositions for simultaneously detecting the activation state of a plurality of proteins in single cells using flow cytometry. The invention further provides methods and compositions of screening for bioactive agents capable of coordinately modulating the activity of a plurality of proteins in single cells. The methods and compositions can be used to determine the protein activation profile of a cell for predicting or diagnosing a disease state, and for monitoring treatment of a disease state.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for detecting the activation state of multiple proteins in single cells
InactiveUS20060073474A1Bioreactor/fermenter combinationsBiological substance pretreatmentsProtein activationBiological activation
The invention provides methods and compositions for simultaneously detecting the activation state of a plurality of proteins in single cells using flow cytometry. The invention further provides methods and compositions of screening for bioactive agents capable of coordinately modulating the activity of a plurality of proteins in single cells. The methods and compositions can be used to determine the protein activation profile of a cell for predicting or diagnosing a disease state, and for monitoring treatment of a disease state.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for detecting receptor-ligand interactions in single cells
InactiveUS7695924B2Bioreactor/fermenter combinationsBiological substance pretreatmentsReceptorActive agent
The invention provides methods and compositions for simultaneously detecting the activation state of a plurality of proteins in single cells using flow cytometry. The invention further provides methods and compositions of screening for bioactive agents capable of coordinately modulating the activity of a plurality of proteins in single cells. The methods and compositions can be used to determine the protein activation profile of a cell for predicting or diagnosing a disease state, and for monitoring treatment of a disease state.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
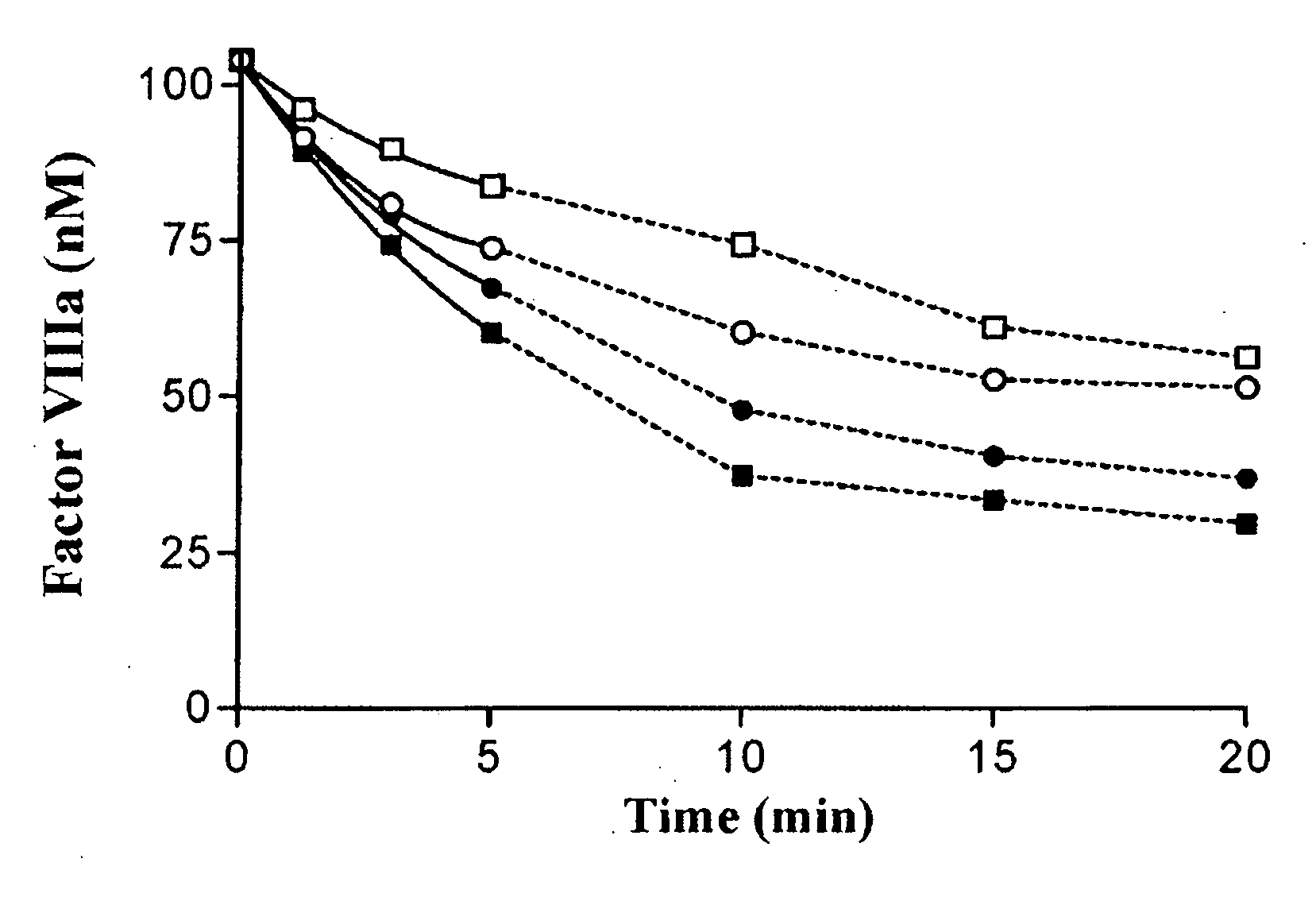
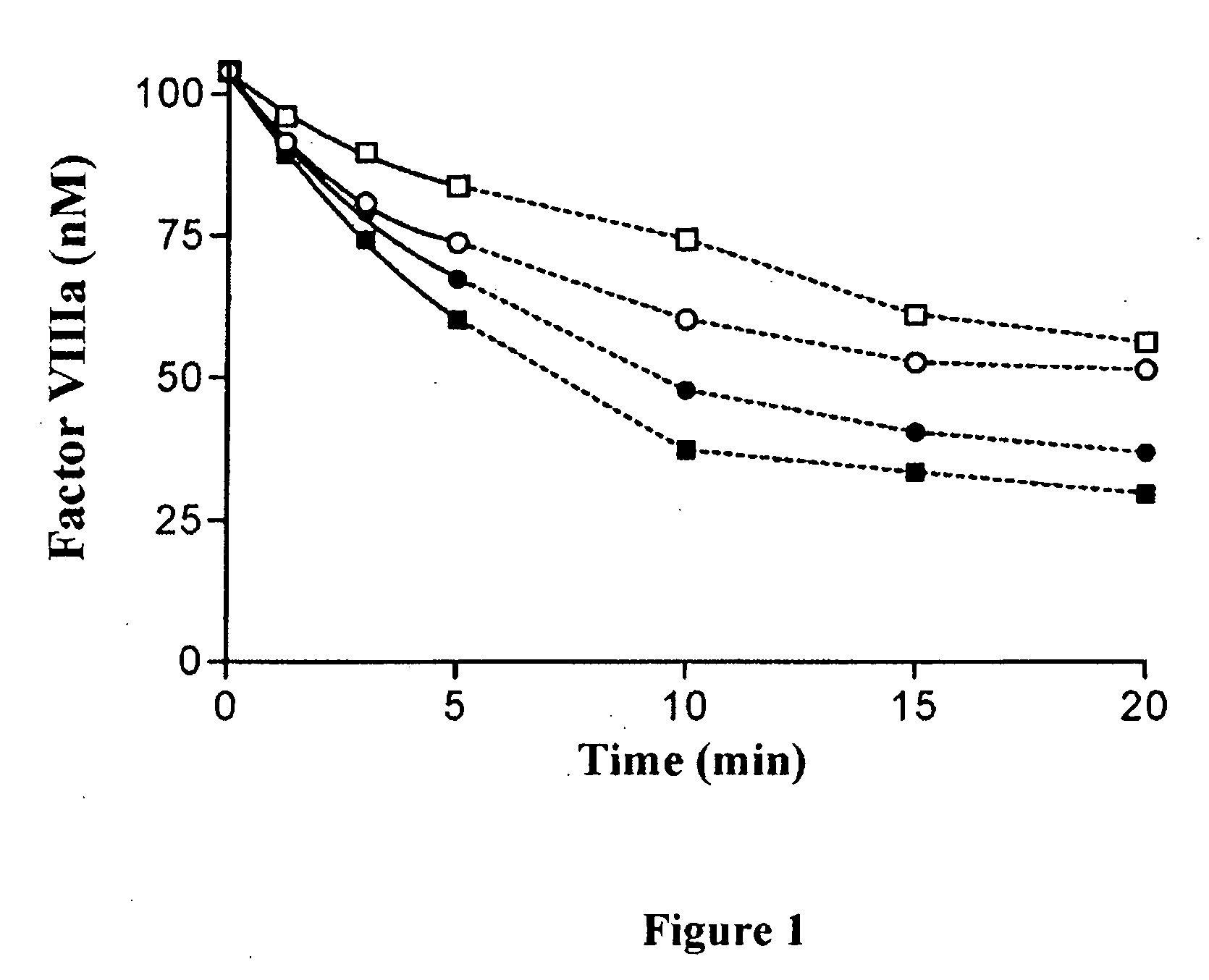
Recombinant factor VIII having reduced inactivation by activated protein C
ActiveUS8183345B2High catalytic efficiencyPromote localizationFactor VIIBacteriaProtein activationClotting disorders
The present invention relates to a recombinant factor VIII that is characterized by one or more mutations within a region surrounding an activated protein C cleavage site, which one or more mutations result in a reduced rate of inactivation by activated protein C. Isolated nucleic acid molecules, recombinant expression vectors, and host cells suitable for expression of the recombinant factor VIII are also disclosed. The recombinant factor VIII can be used for the treatment of clotting disorders, such as hemophilia A.
Owner:UNIVERSITY OF ROCHESTER
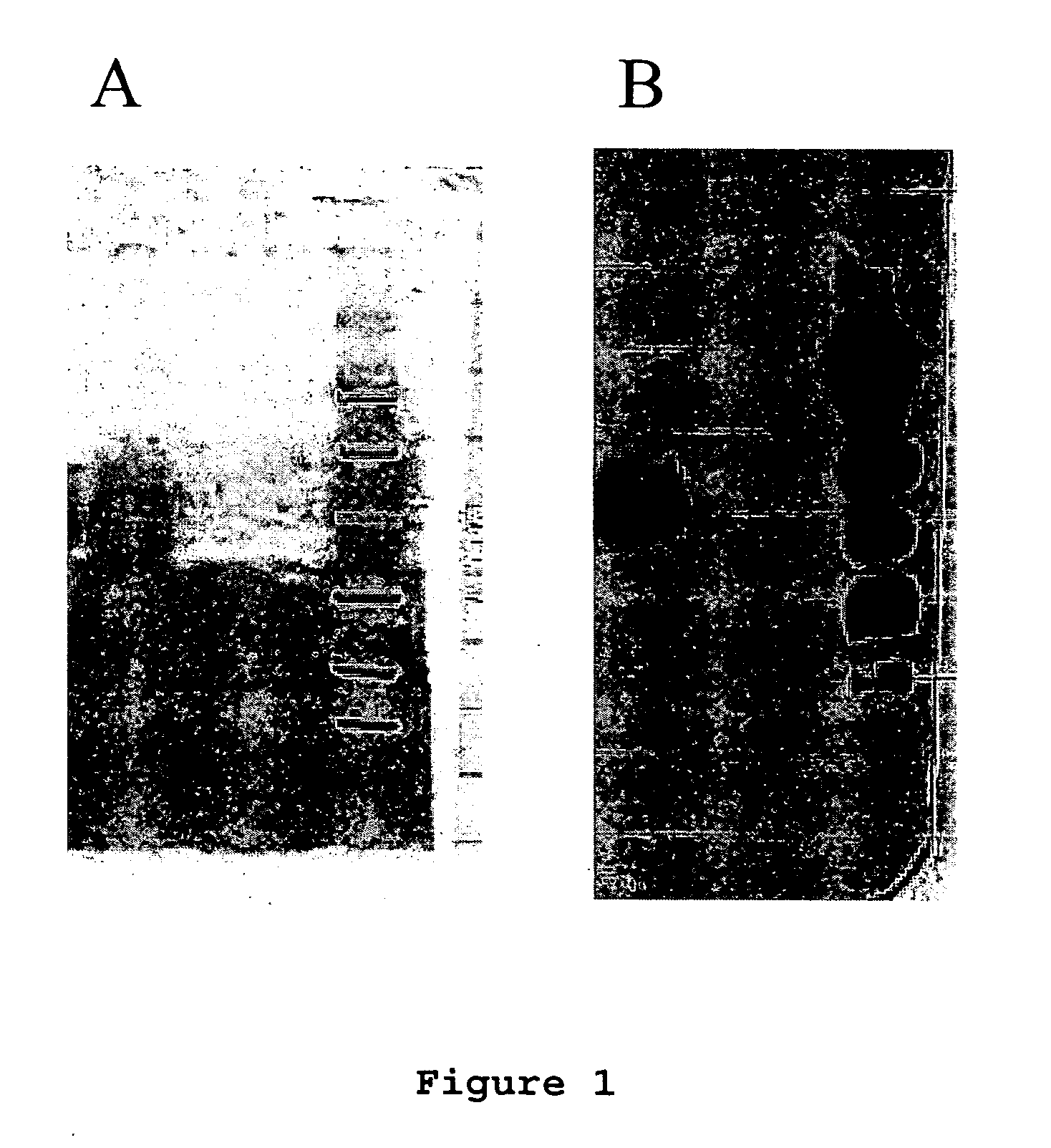
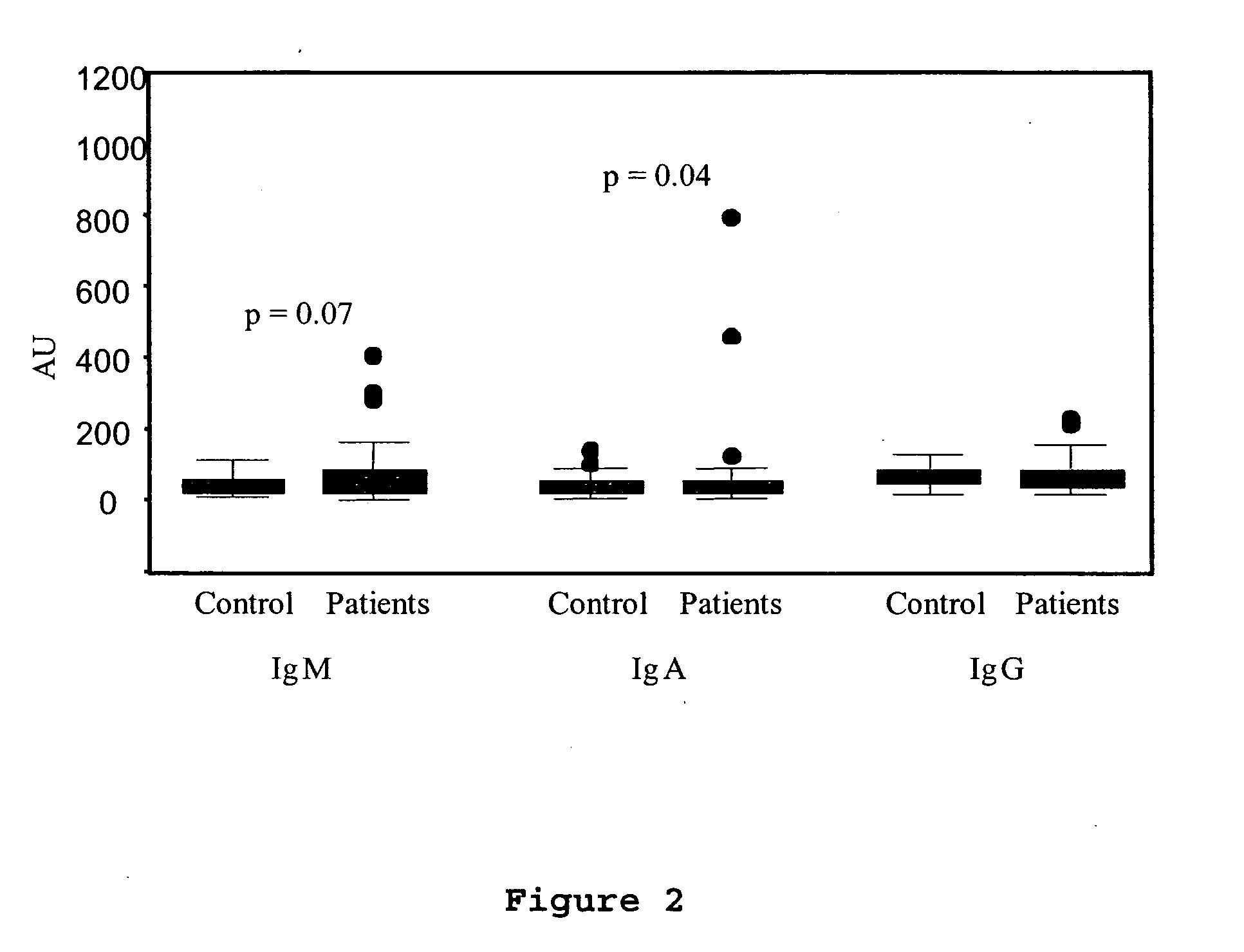
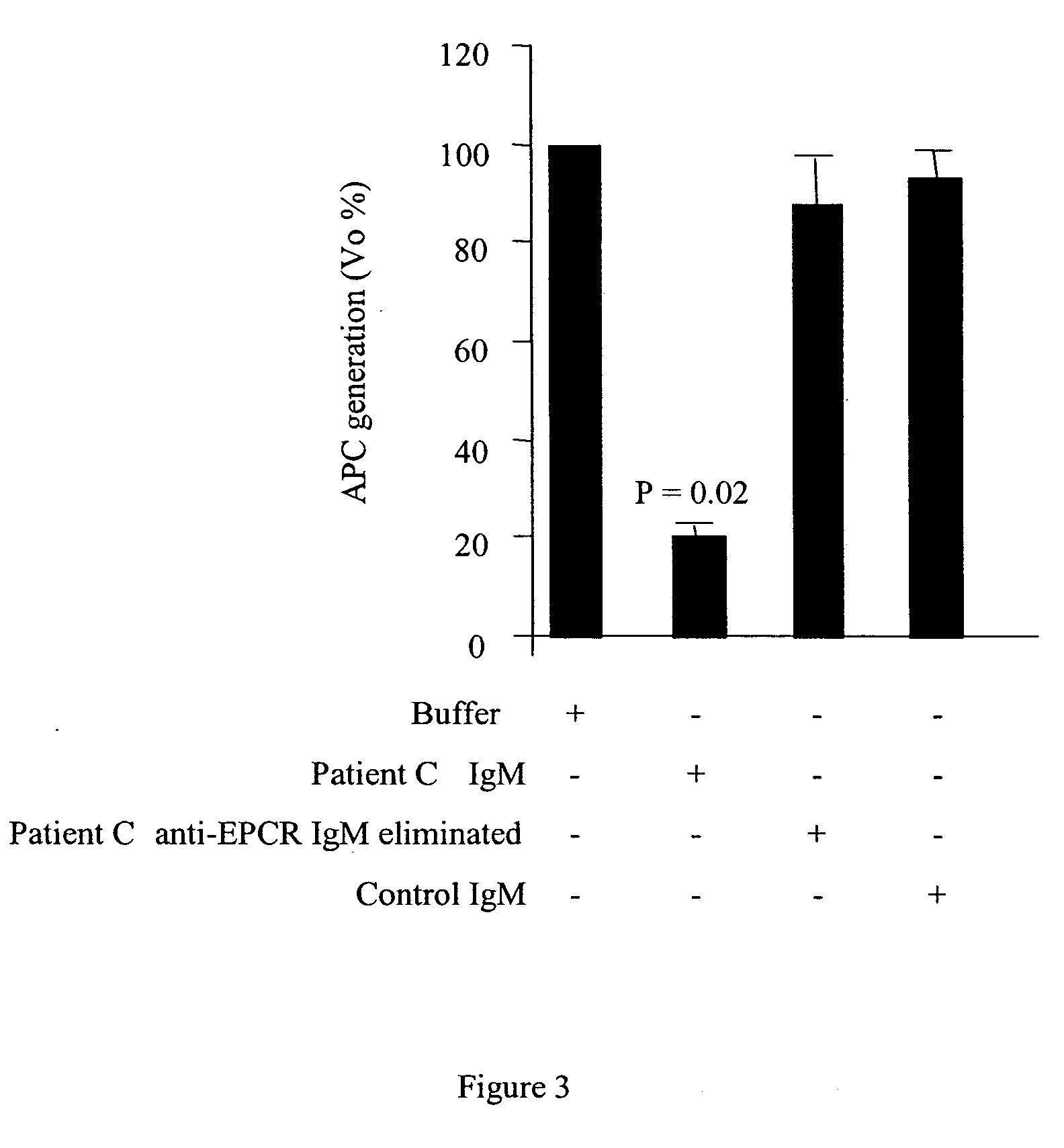
Method for assessing risk of and predisposition to development of a pathology related to the presence of anti-epcr autoantibodies
The present invention relates to a method for detecting the presence of high levels of autoantibodies against protein C / activated protein C endothelial receptor (EPCR). The invention is characterised in that it comprises in vitro detection and quantification of anti-EPCR autoantibodies in a sample.
Owner:PROYECTO DE BIOMEDICINA CIMA
Methods and compositions for detecting receptor-ligand interactions in single cells
InactiveUS20070196870A1Microbiological testing/measurementPreparing sample for investigationProtein activationActive agent
The invention provides methods and compositions for simultaneously detecting the activation state of a plurality of proteins in single cells using flow cytometry. The invention further provides methods and compositions of screening for bioactive agents capable of coordinately modulating the activity of a plurality of proteins in single cells. The methods and compositions can be used to determine the protein activation profile of a cell for predicting or diagnosing a disease state, and for monitoring treatment of a disease state.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Preparation method of formaldehyde-free water-resistant protein adhesive
InactiveCN103937441ALow costIncrease crosslink densityNon-macromolecular adhesive additivesProtein adhesivesPROTEIN S HEERLENProtein activation
The invention relates to a preparation method of a formaldehyde-free water-resistant protein adhesive. The main preparation steps are as follows: 1) activating a protein raw material: adding the protein-containing raw material and absolute ethanol, stirring, further adding an alkaline solution, and heating to 45-80 DEG C for reaction; cooling to room temperature and adding a reducing agent; and adjusting to neutral, and evaporating to dryness to obtain activated protein powder; and 2) preparing the protein adhesive: adding the activated protein powder prepared in the step 1, water, an acid-adjusting reagent, an enhancer and a preservative into a reactor, reacting at the temperature of 30-50 DEG C, and reducing to room temperature to obtain the protein adhesive. The preparation method is characterized in that a special protein activation process is adopted to enable proteins to expose active groups thereof and utilize a variety of active functional groups of the proteins to the greater extent; the preparation process of the adhesive is simple and has the advantages of good reproductivity, low cost, no formaldehyde and good water resistance; and a hot pressing process is the same with an existing urea-formaldehyde resin pressure plate process, on the basis of not changing and increasing the hot pressing process and equipment, the production of formaldehyde-free artificial plates can be realized, and the market prospects are greater in comparison with the existing protein adhesive in industrial production.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
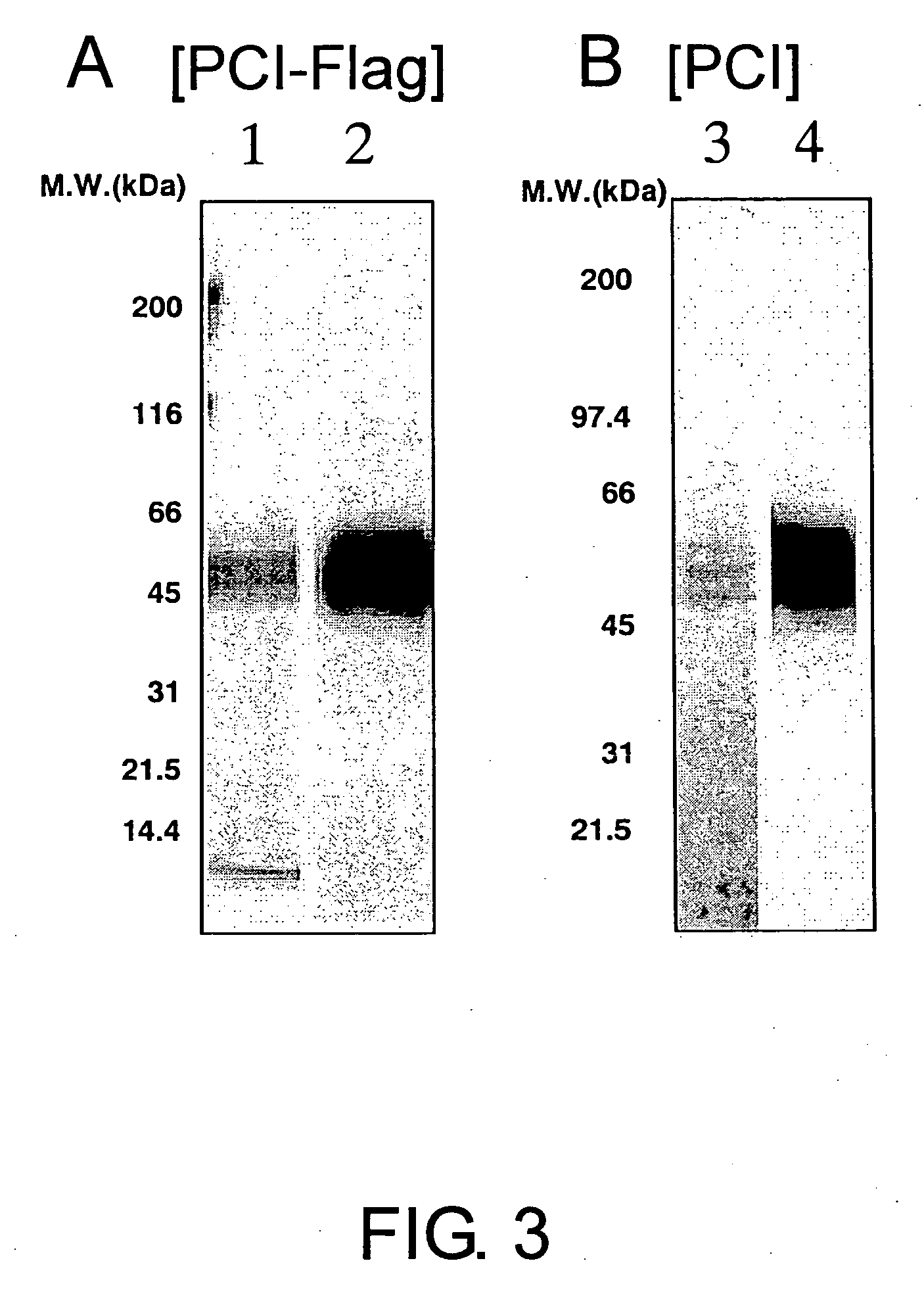
Anti-pci neutralizing antibody
InactiveUS20060167230A1Increase productionHigh activityAntibacterial agentsAntipyreticProtein C inhibitorTherapeutic effect
The present invention provides anti-PCI antibodies having Protein C inhibitor (PCI)-neutralizing activity, and the uses thereof. Through the generation and screening of anti-PCI antibodies, the inventors successfully isolated anti-PCI antibodies which inhibit PCI's inhibitory effect on the production and activity of activated Protein C (aPC). The antibodies of the present invention suppress PCI's inhibitory effect on aPC production and / or the aPC inactivation by PCI, and thus can be used to maintain aPC activity and sustain the effects of aPC physiological activities, such as suppression of the activation of blood coagulation system and anti-inflammatory functions. The present invention also provides uses of the antibodies of the present invention in treating diseases such as thrombosis and sepsis using aPC. In treatments by aPC administration, the therapeutic effect of aPC can be sustained by administering an antibody of the present invention. The antibodies of the present invention can be used in the treatment and prevention of diseases such as thrombosis and sepsis.
Owner:CHUGAI PHARMA CO LTD
Apc non-neutralizing anti-body
ActiveUS20060121022A1High activityPotentiate actionAntibacterial agentsImmunoglobulins against animals/humansDiseaseAntiendomysial antibodies
The present invention provides anti-aPC antibodies that suppress the inactivation of activated protein C (aPC), and uses thereof. The present inventors screened anti-aPC antibodies, and succeeded in isolating anti-aPC antibodies comprising the activity of suppressing aPC inactivation in blood. The antibodies of the present invention can be used to maintain aPC activity by suppressing aPC inactivation, and can thus be used to sustain aPC bioactivities, such as the activity of suppressing activation of the blood coagulation system, and anti-inflammatory activity. In addition, the present invention provides uses of the antibodies of the present invention in aPC therapy for diseases such as thrombosis and sepsis. The therapeutic effect of aPC can be prolonged in treatment that uses aPC administration by allowing an antibody of the present invention to bind with aPC. The antibodies of the present invention can be used in the treatment and prevention of diseases such as thrombosis and sepsis.
Owner:CHUGAI PHARMA CO LTD
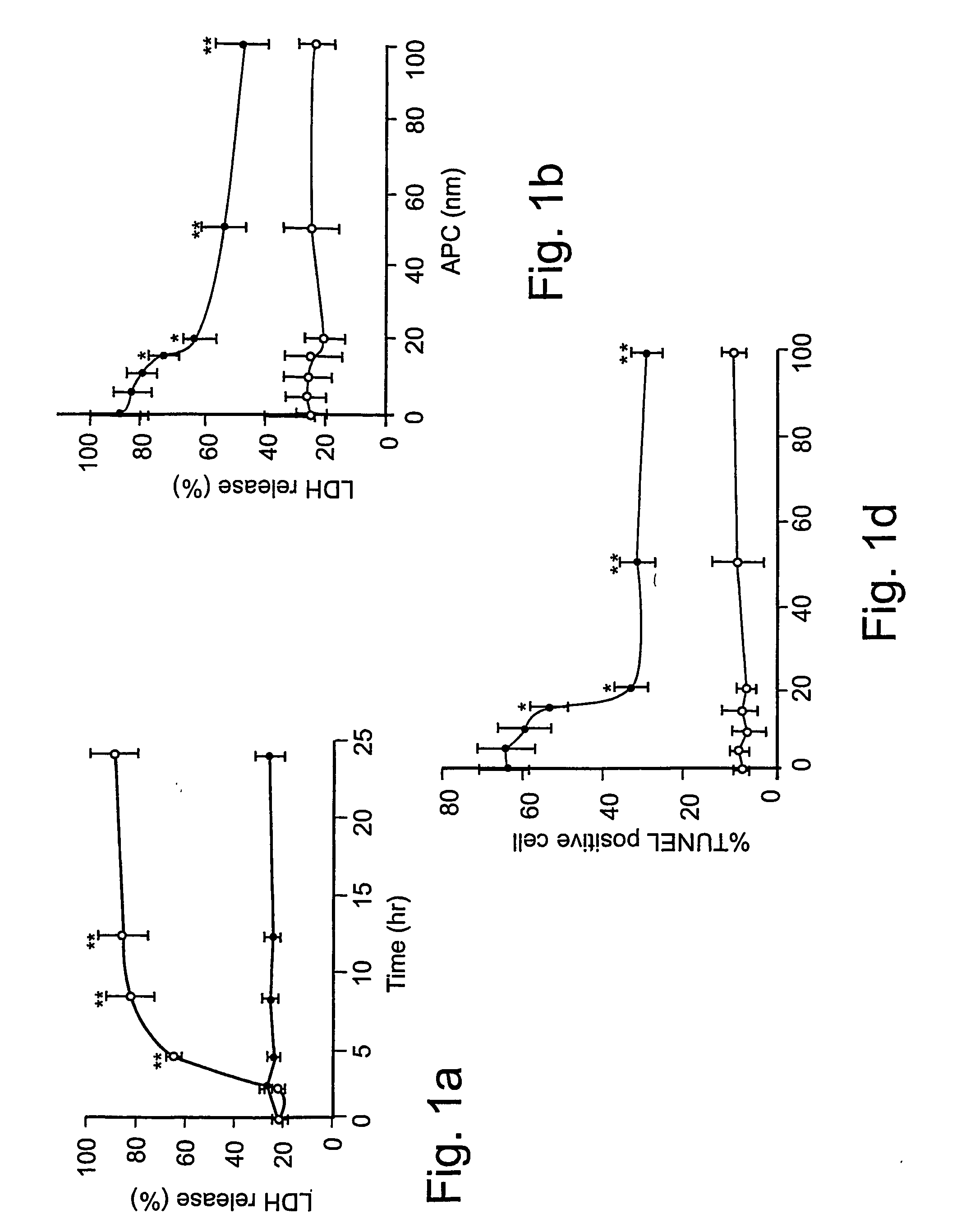
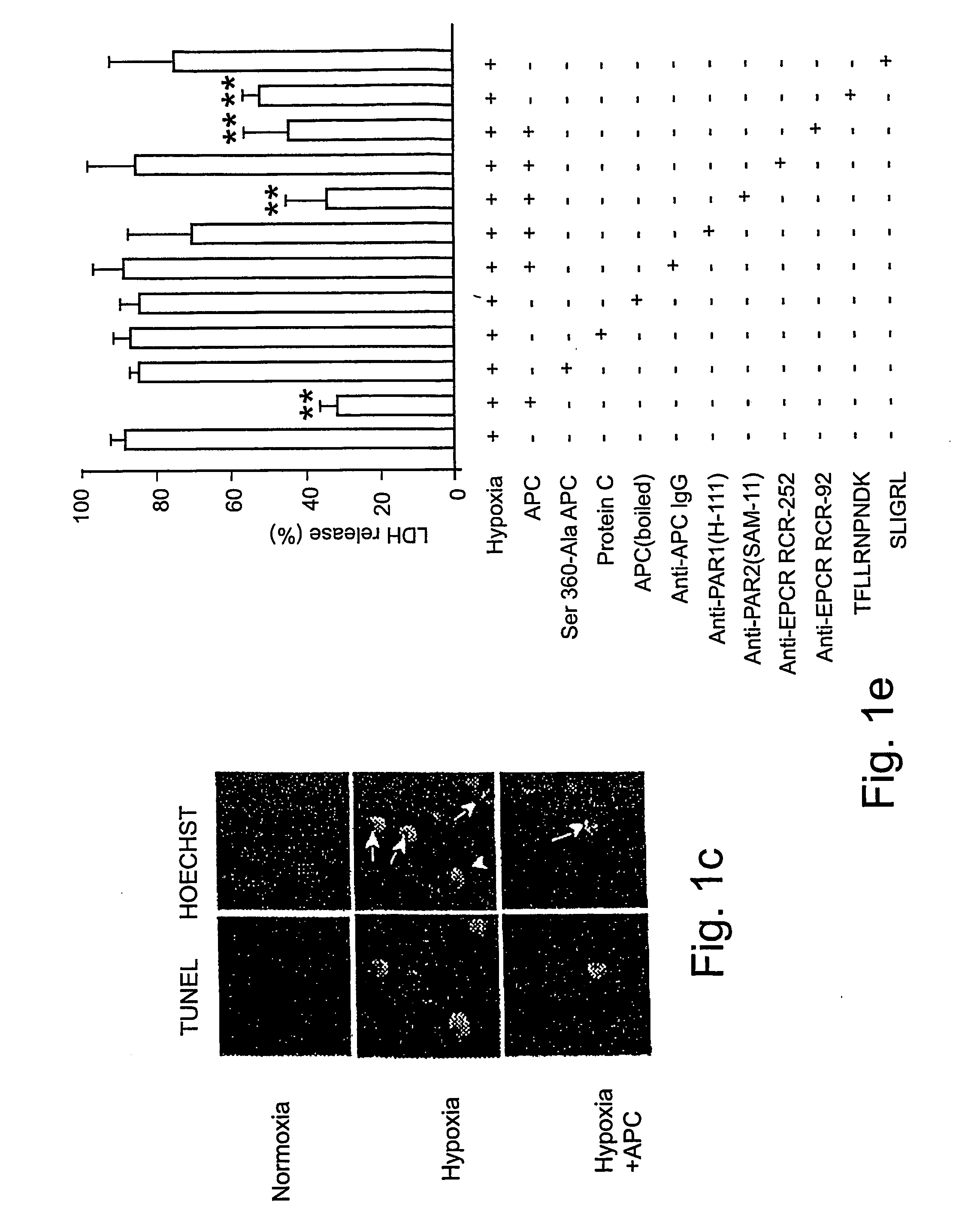
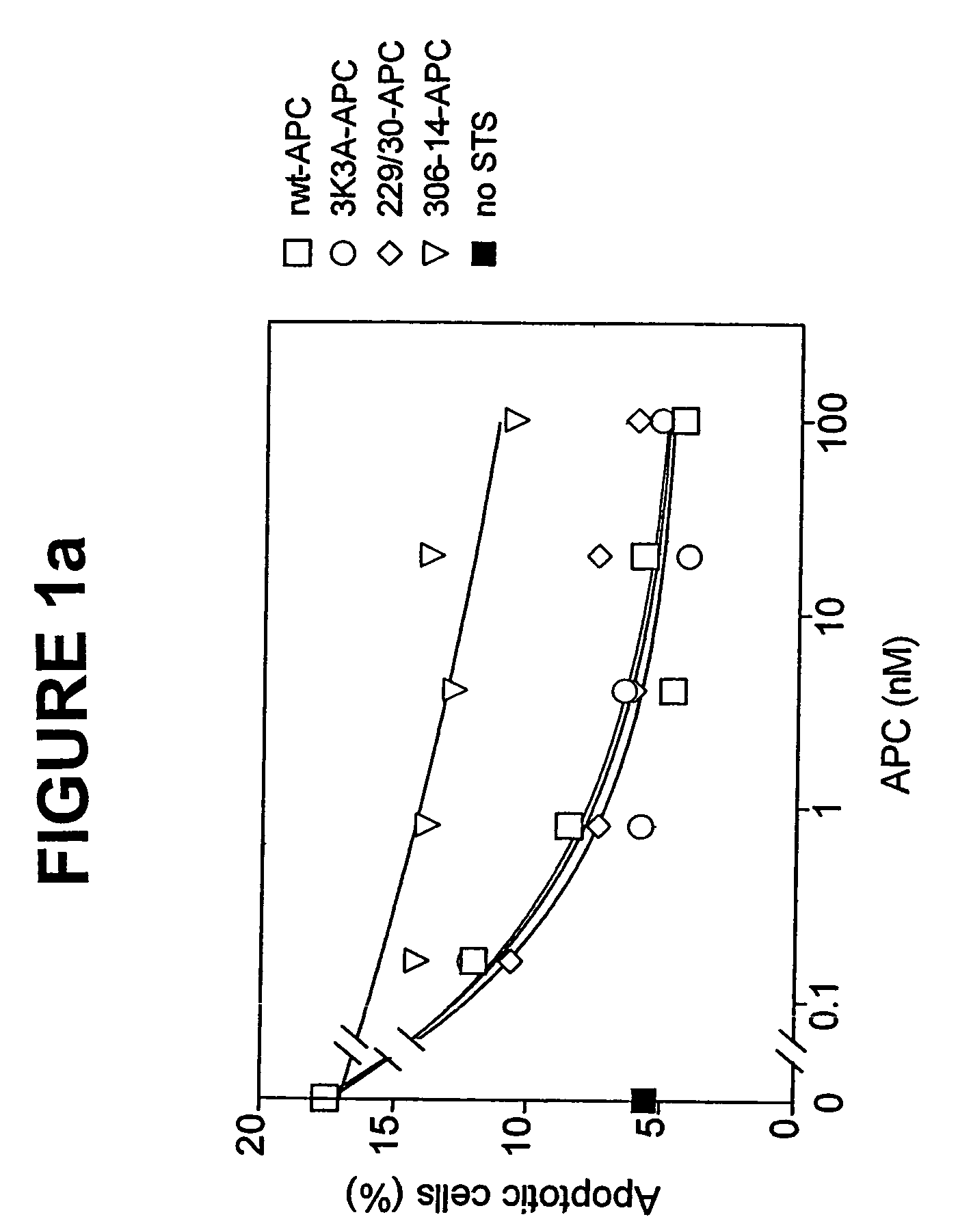
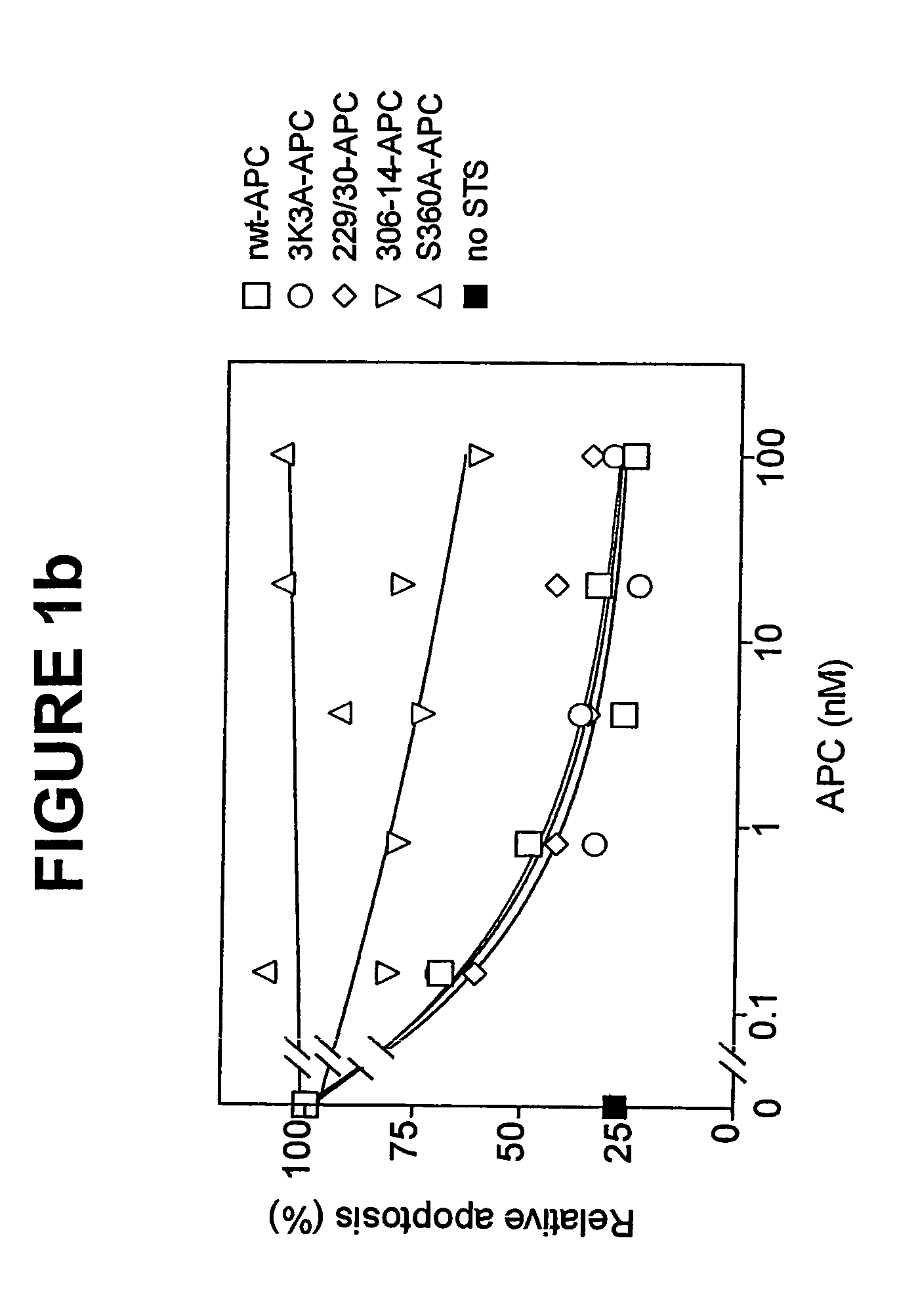
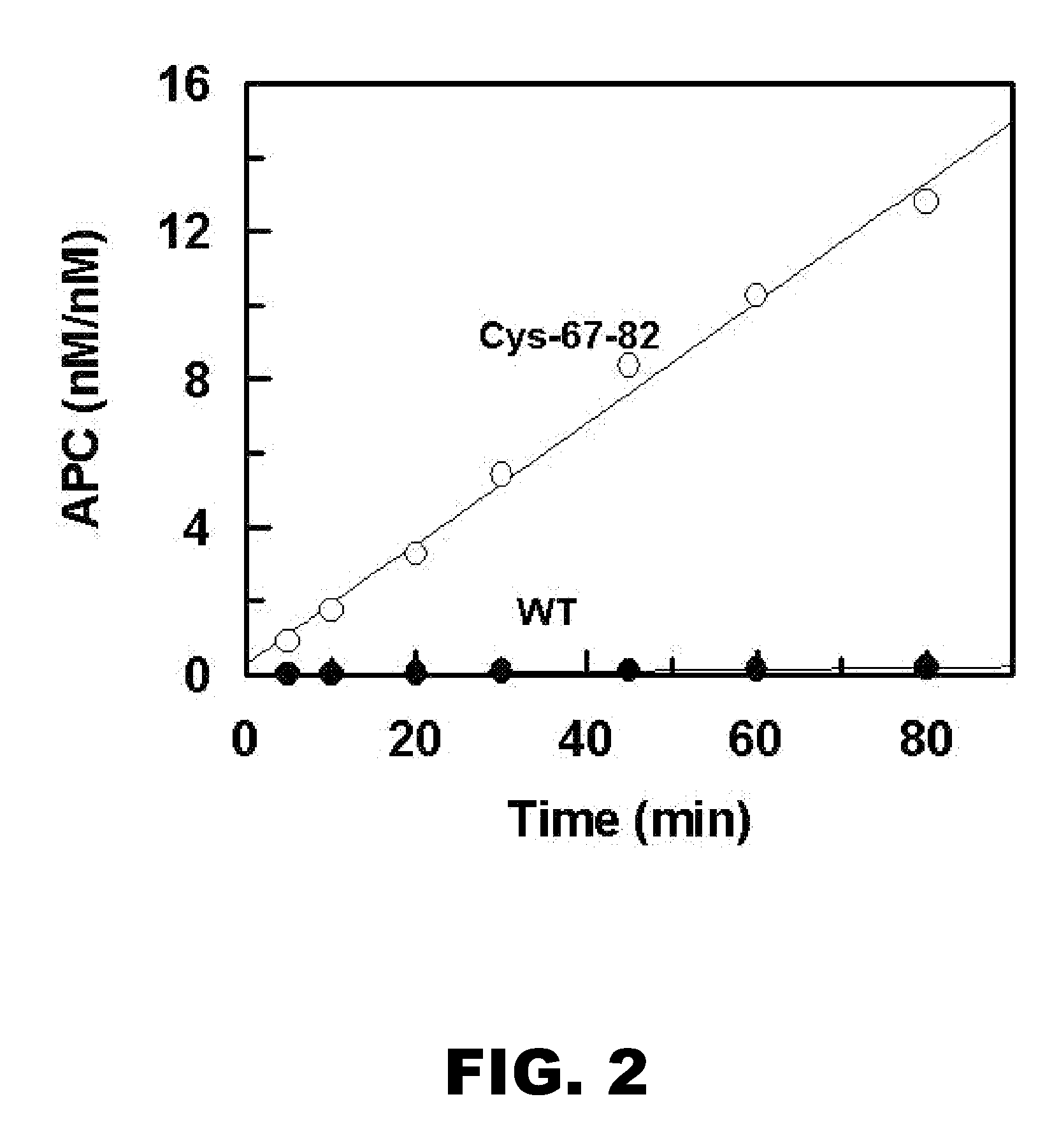
Neuroprotective activity of activated protein c independent of its anticoagulant activity
InactiveUS20070142272A1Enhancing neuroprotectionPromote cell survivalCompound screeningNervous disorderNervous systemInhibitor of apoptosis
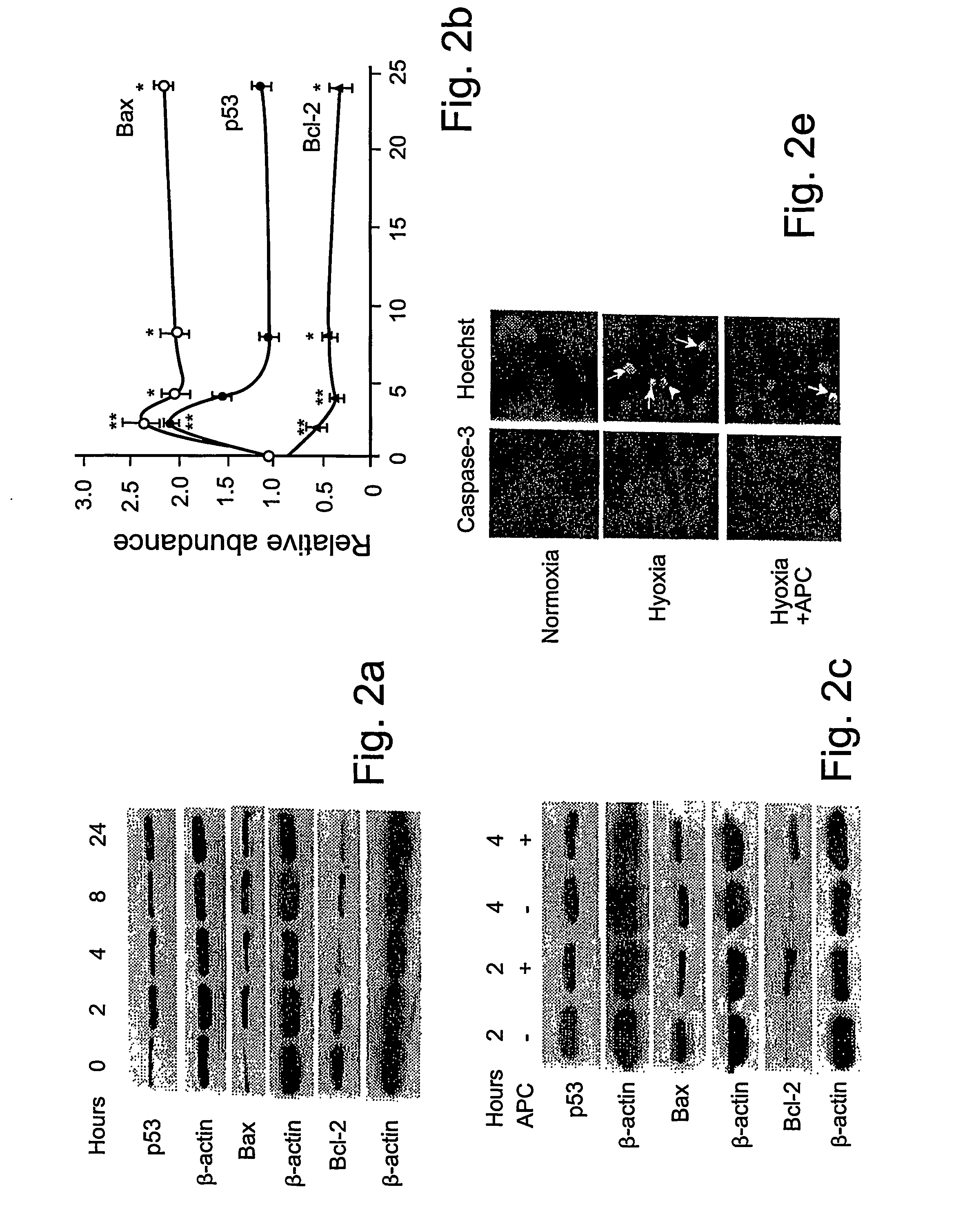
Activated protein C (APC), prodrug, and / or a variant thereof may be used as an inhibitor of apoptosis or cell death and / or a cell survival factor, especially for stressed or injured cells or tissues of the nervous system including subjects with neurode-generative disorders. Novel biological functions (e.g., neuroprotection) can be independent or separated from inhibition of clotting or inflammation, and other biological properties of APC (e.g., antithrombotic activity, ability to reduce NFκB-regulated gene expression). It can be used in the treatment of disease or other pathological conditions by at least inhibiting the p53-dependent and / or caspase-3-dependent pro-apoptotic signaling pathways in stressed or injured cells. Thus, APC, prodrugs, and variants thereof (e.g., APC protease domain mutants with reduced anti-coagulant activity) are prototypes of a class of agents for preventing apoptosis or cell death and / or promoting cell survival by direct action on brain cells. New protein C and / or APC variants with reduced anticoagulant activity may be selected thereby.
Owner:UNIVERSITY OF ROCHESTER +2
Methods and compositions for detecting receptor-ligand interactions in single cells
InactiveUS20070196868A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseProtein activation
The invention provides methods and compositions for simultaneously detecting the activation state of a plurality of proteins in single cells using flow cytometry. The invention further provides methods and compositions of screening for bioactive agents capable of coordinately modulating the activity of a plurality of proteins in single cells. The methods and compositions can be used to determine the protein activation profile of a cell for predicting or diagnosing a disease state, and for monitoring treatment of a disease state.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
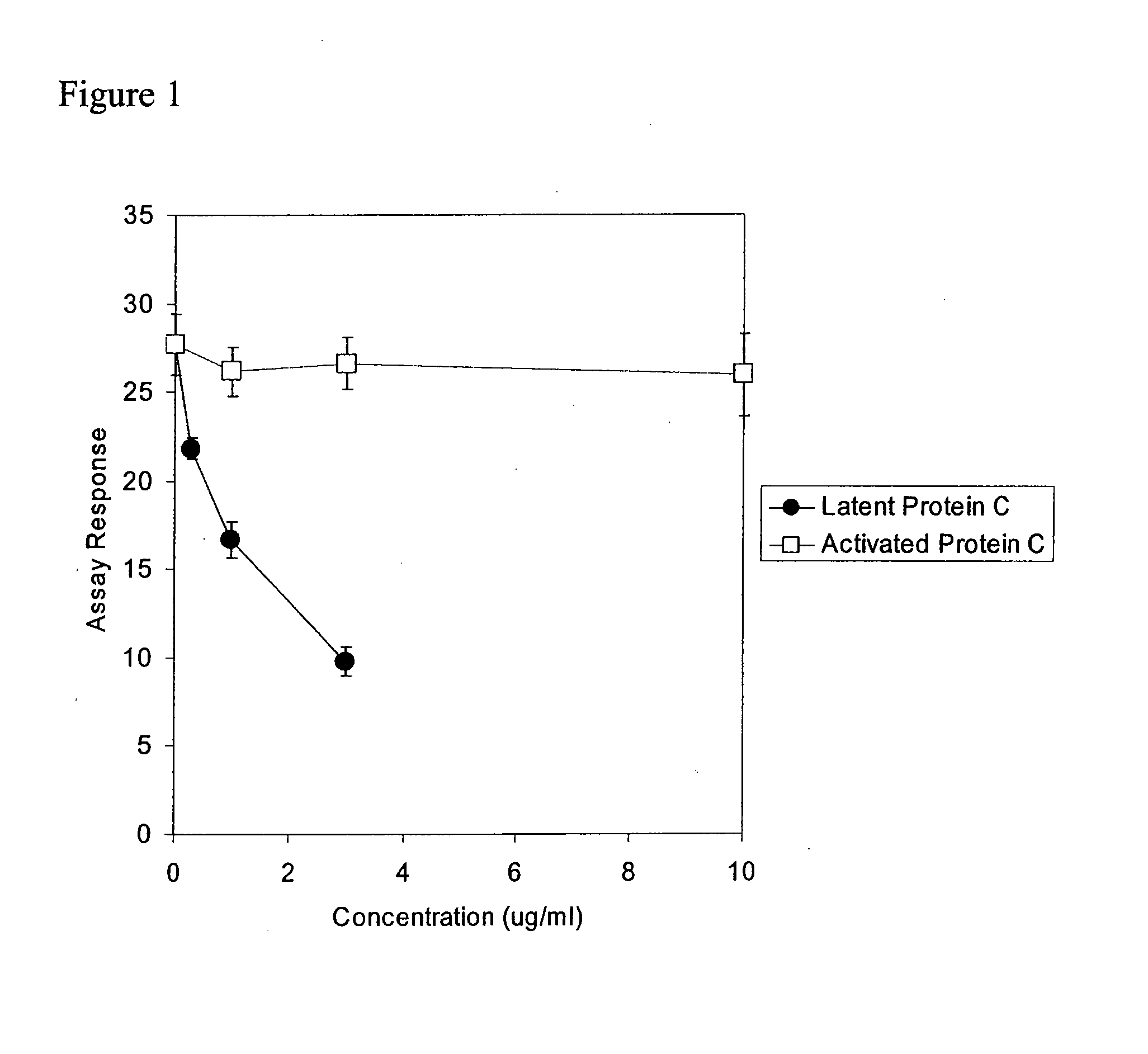
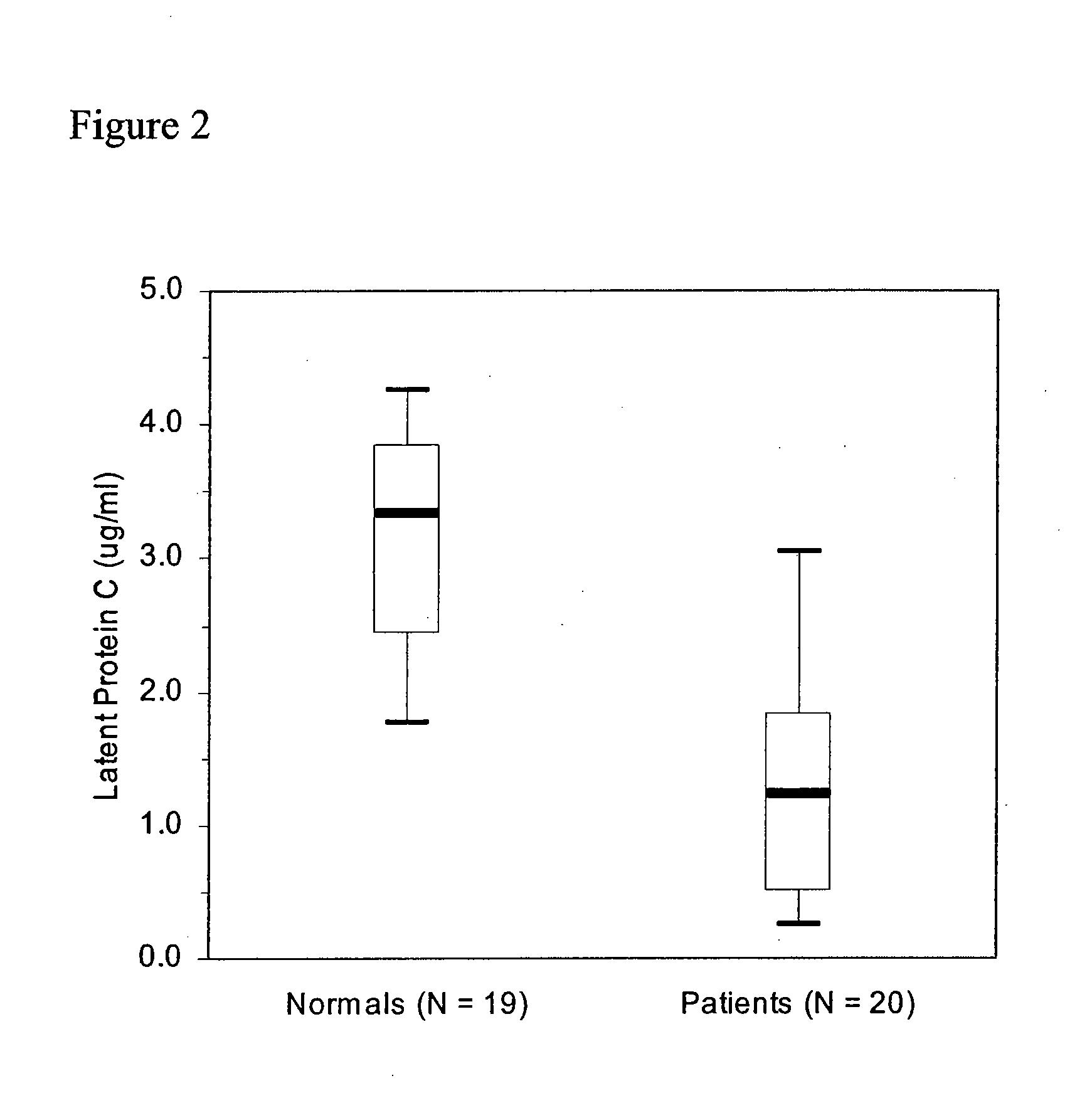
Latent protein c assays and their uses for diagnosis and/or prognosis in systemic inflammatory response syndromes
ActiveUS20070172906A1Immunoglobulins against blood coagulation factorsMaterial thermal conductivityMeasurement testTest sample
Owner:BIOSITE INC
Recombinant factor viii having reduced inactivation by activated protein c
ActiveUS20090118185A1High catalytic efficiencyPromote localizationFactor VIIFungiProtein activationFactor ii
The present invention relates to a recombinant factor VIII that is characterized by one or more mutations within a region surrounding an activated protein C cleavage site, which one or more mutations result in a reduced rate of inactivation by activated protein C. Isolated nucleic acid molecules, recombinant expression vectors, and host cells suitable for expression of the recombinant factor VIII are also disclosed. The recombinant factor VIII can be used for the treatment of clotting disorders, such as hemophilia A.
Owner:UNIVERSITY OF ROCHESTER
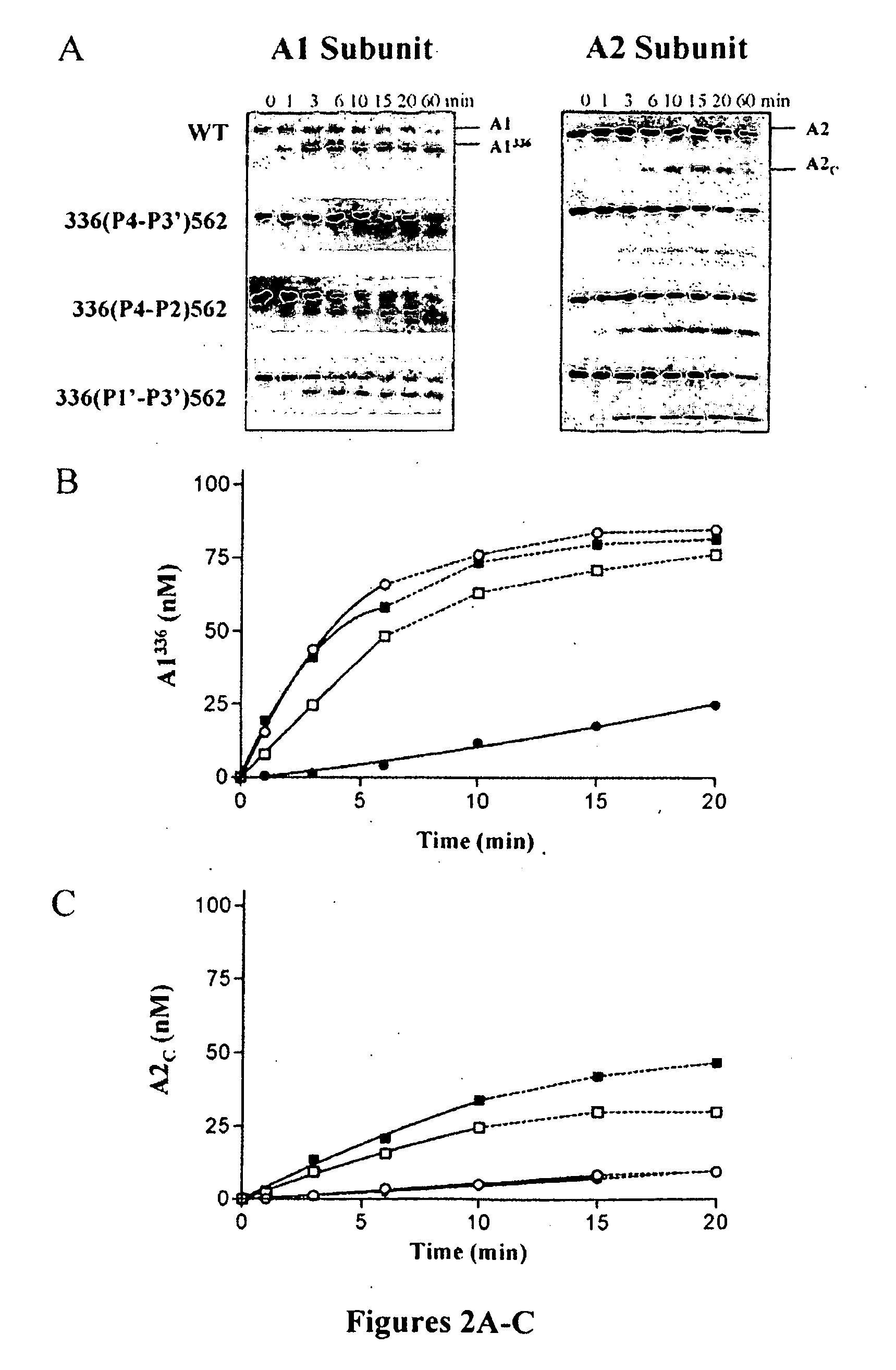
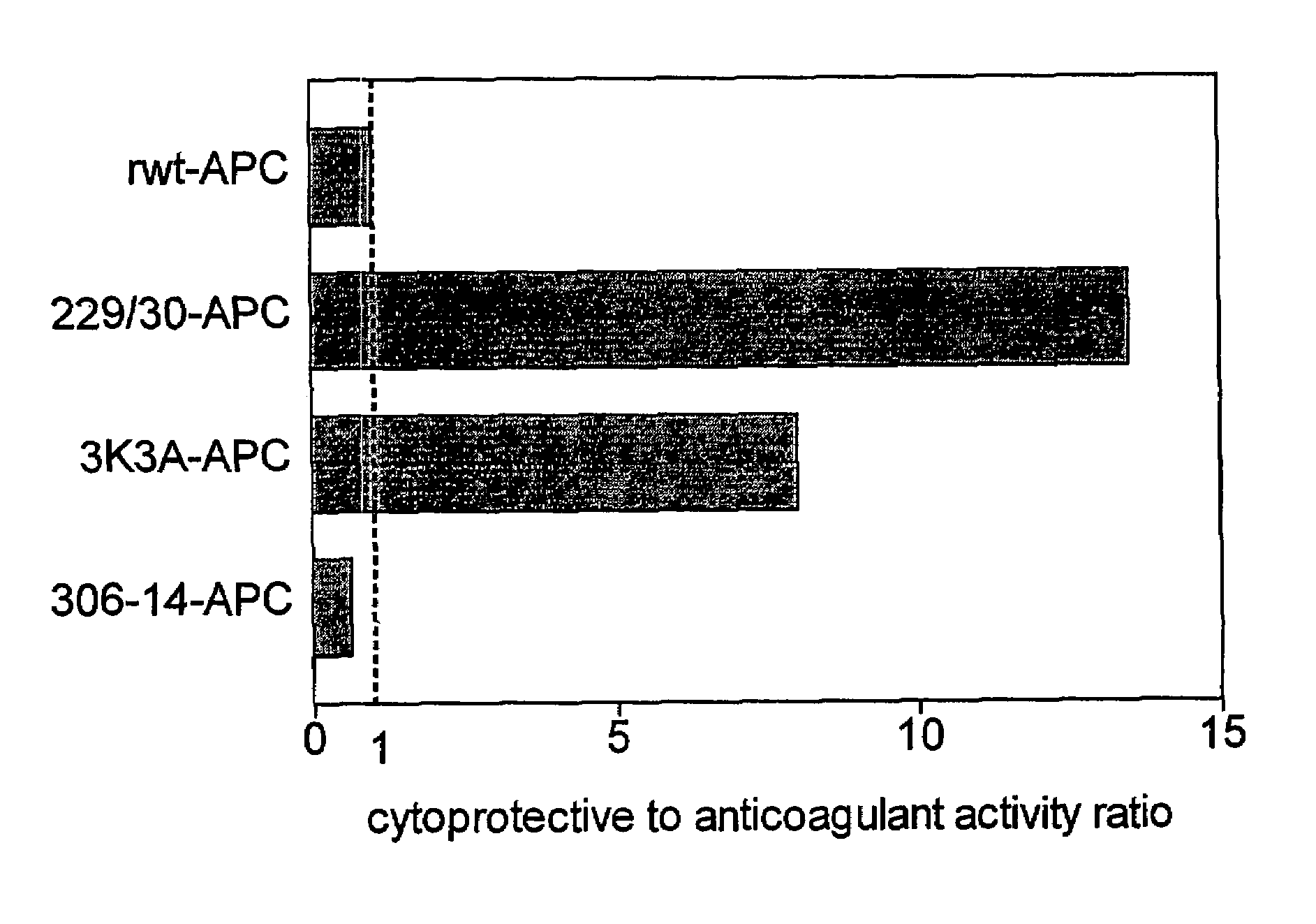
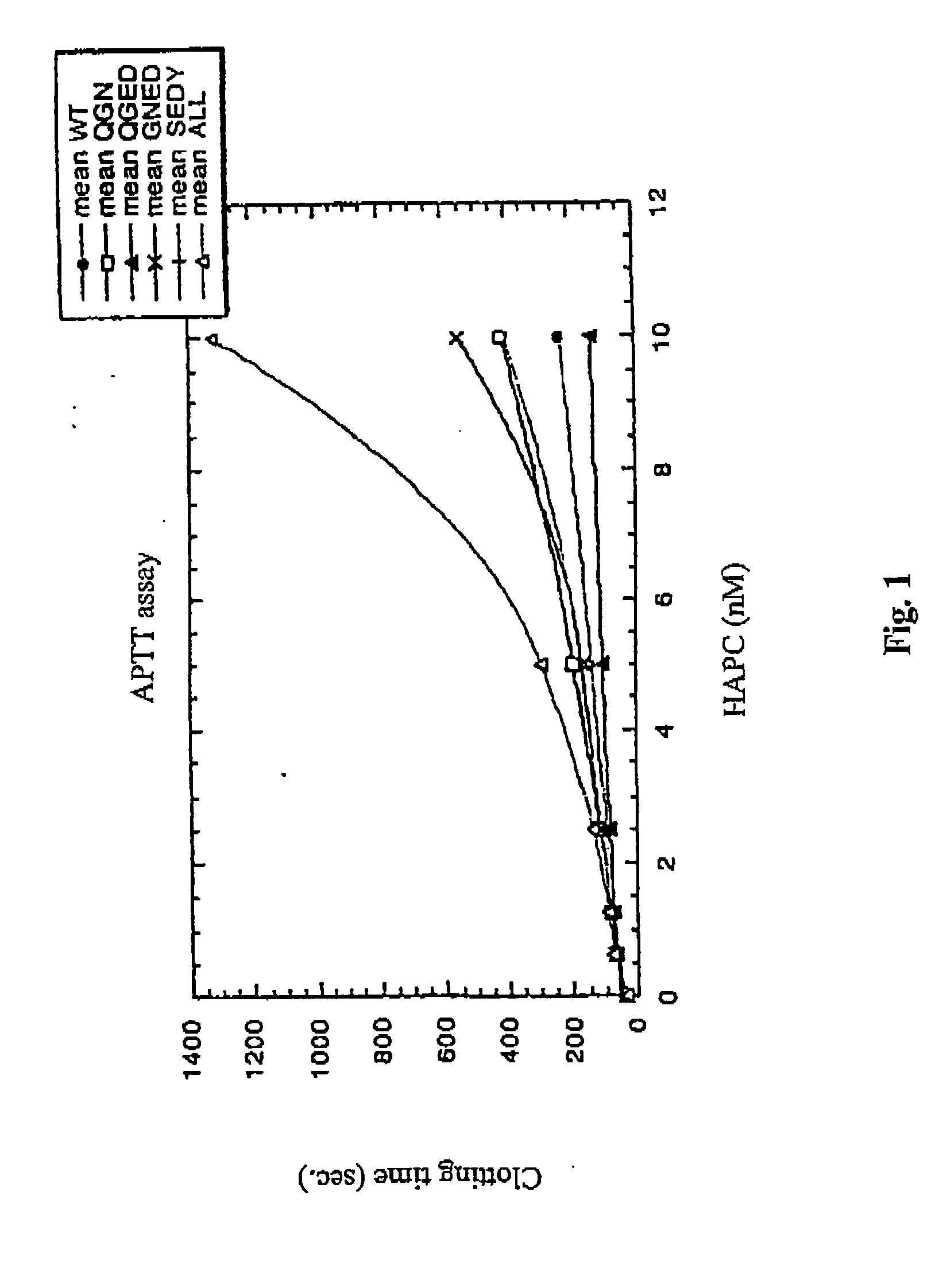
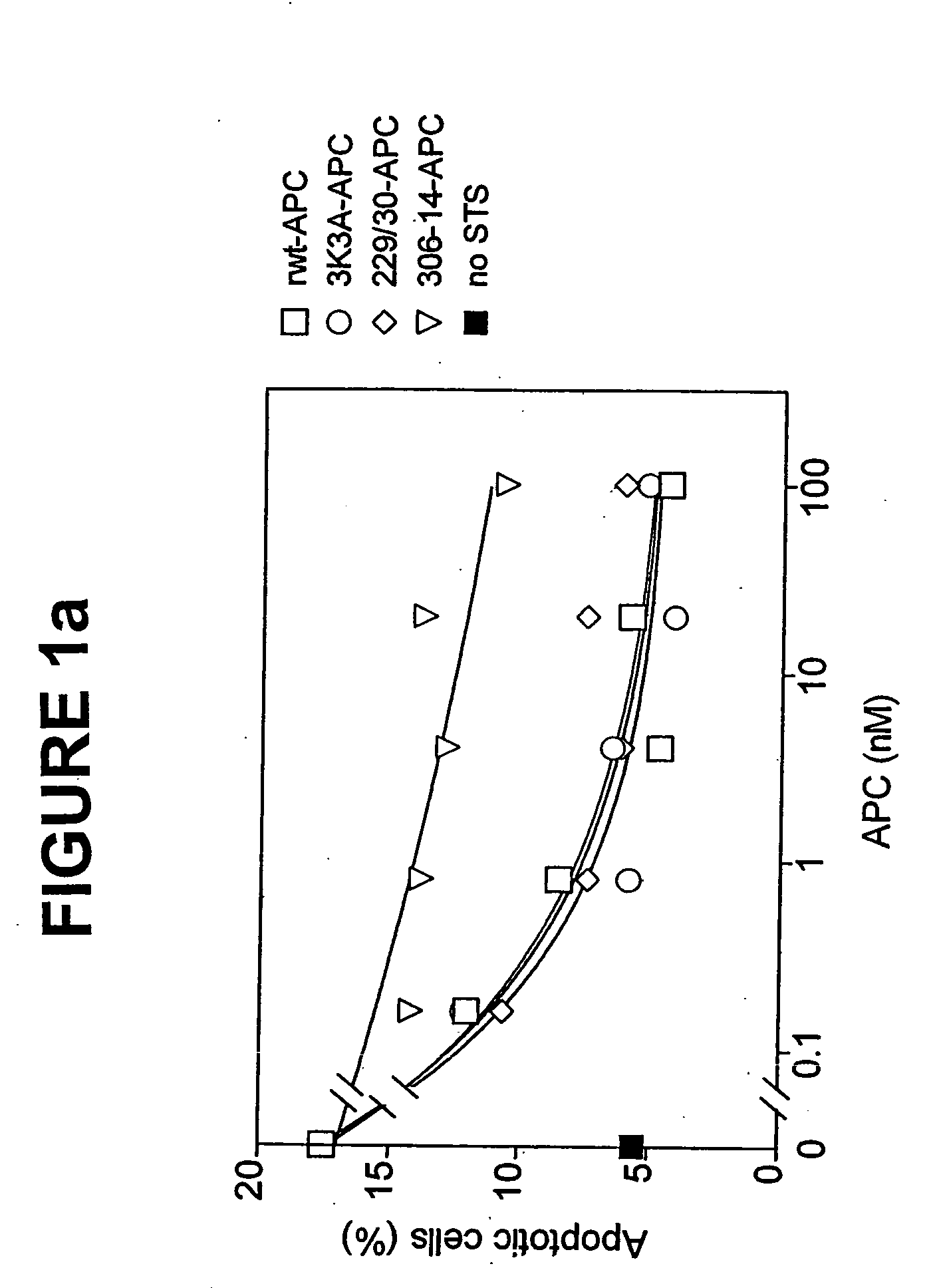
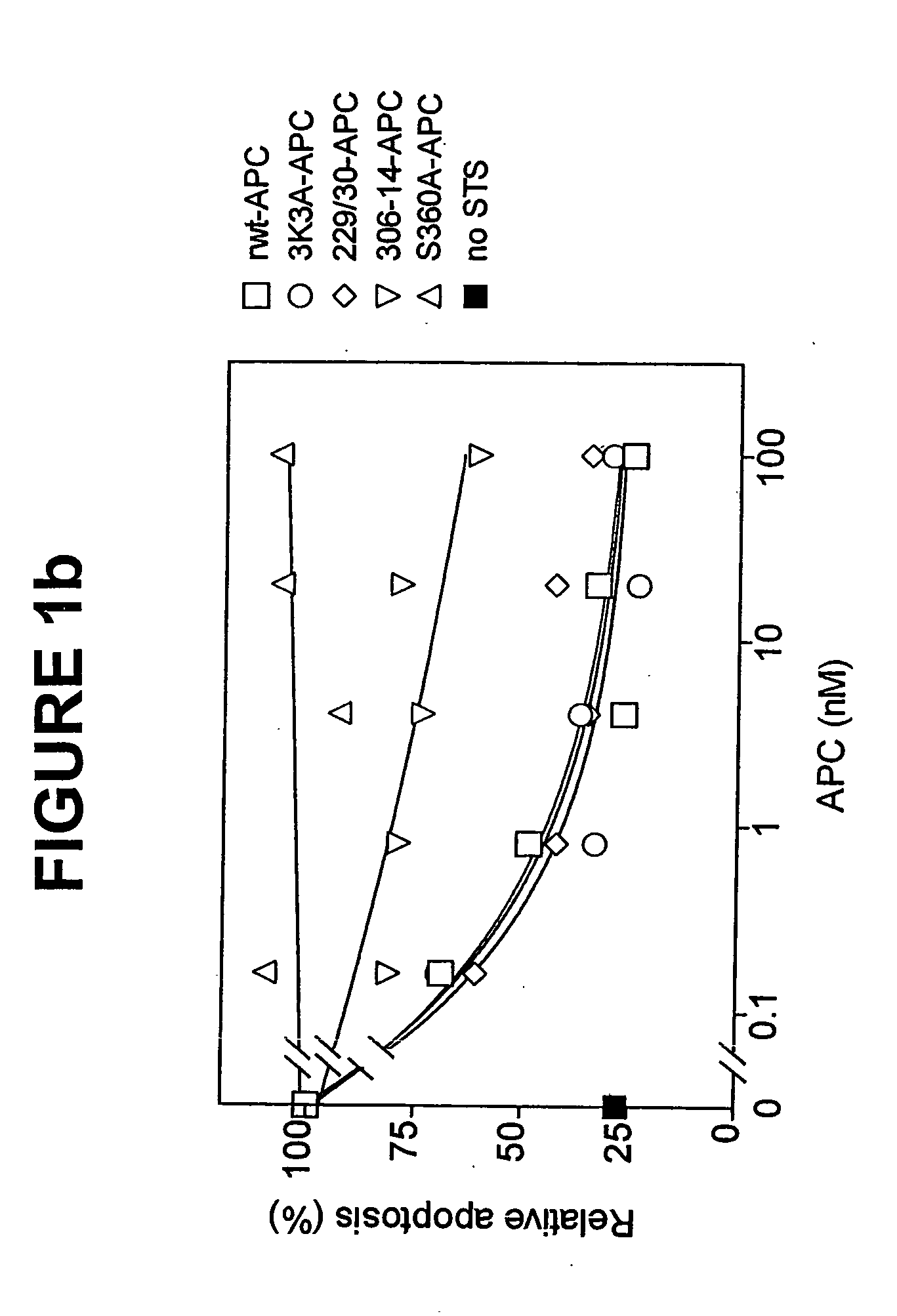
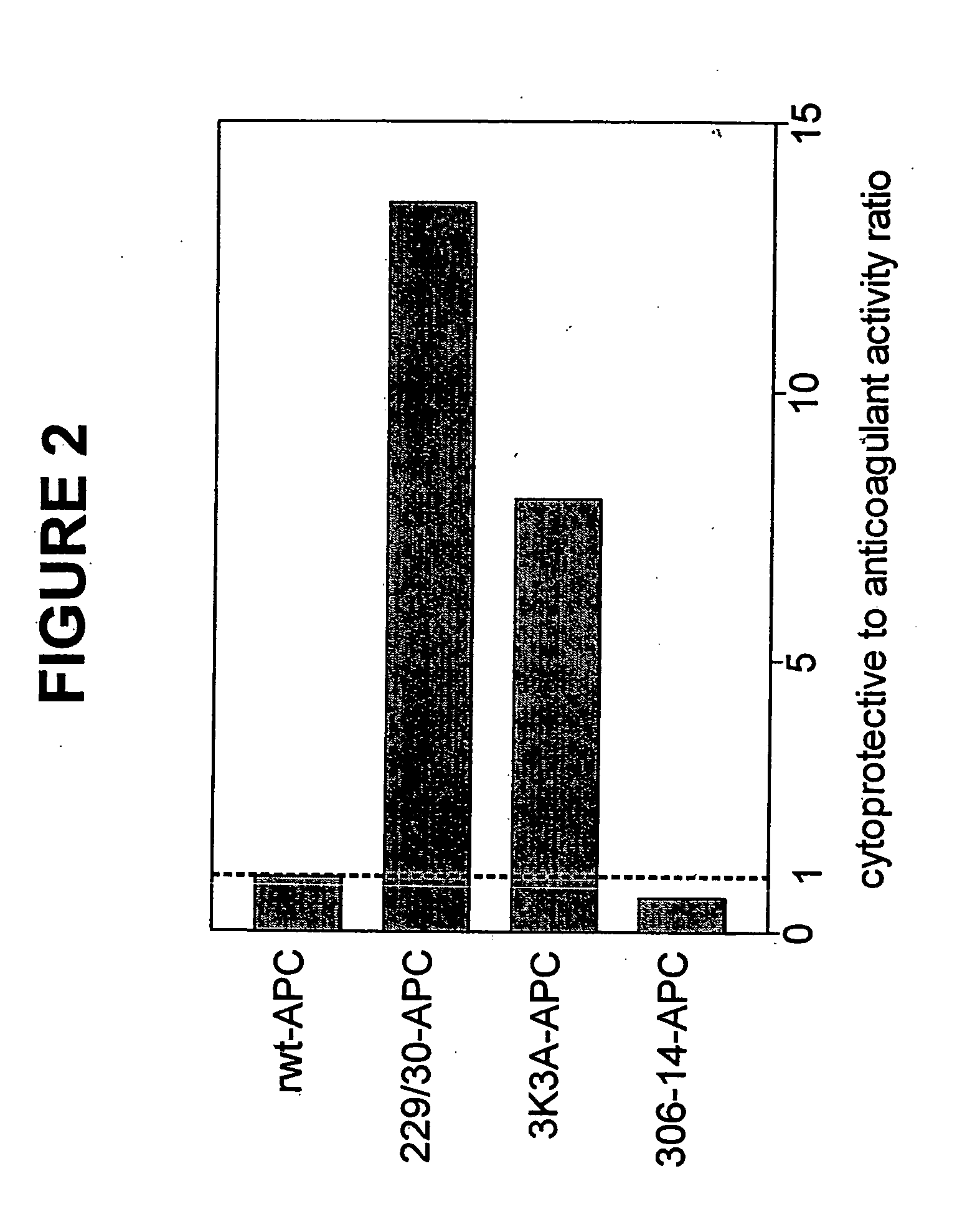
Activated protein C variants with normal cytoprotective activity but reduced anticoagulant activity
ActiveUS7498305B2Reduced activityReduce bleeding riskAntibacterial agentsNervous disorderReperfusion injuryApoptosis
Variants (mutants) of recombinant activated protein C (APC) or recombinant protein C (prodrug, capable of being converted to APC) that have substantial reductions in anticoagulant activity but that retain normal levels of anti-apoptotic activity are provided. Two examples of such recombinant APC mutants are KKK191-193AAA-APC and RR229 / 230M-APC. APC variants and prodrugs of the invention have the desirable property of being cytoprotective (anti-apoptotic effects), while having significantly reduced risk of bleeding. The invention also provides a method of using the APC variants or prodrugs of the invention to treat subjects who will benefit from APC's cytoprotective activities that are independent of APC's anticoagulant activity. These subjects include patients at risk of damage to blood vessels or tissue in various organs caused, at least in part, by apoptosis. At risk patients include, for example, those suffering (severe) sepsis, ischemia / reperfusion injury, ischemic stroke, acute myocardial infarction, acute or chronic neurodegenerative diseases, or those undergoing organ transplantation or chemotherapy, among other conditions. Methods of screening for variants of recombinant protein C or APC that are useful in accordance with the invention are also provided.
Owner:THE SCRIPPS RES INST
Method for diagnosing an increased risk for thrombosis or a genetic defect causing thrombosis and kit for use with the same
InactiveUS6518016B1Immunoglobulins against blood coagulation factorsSugar derivativesBinding siteProtein activation
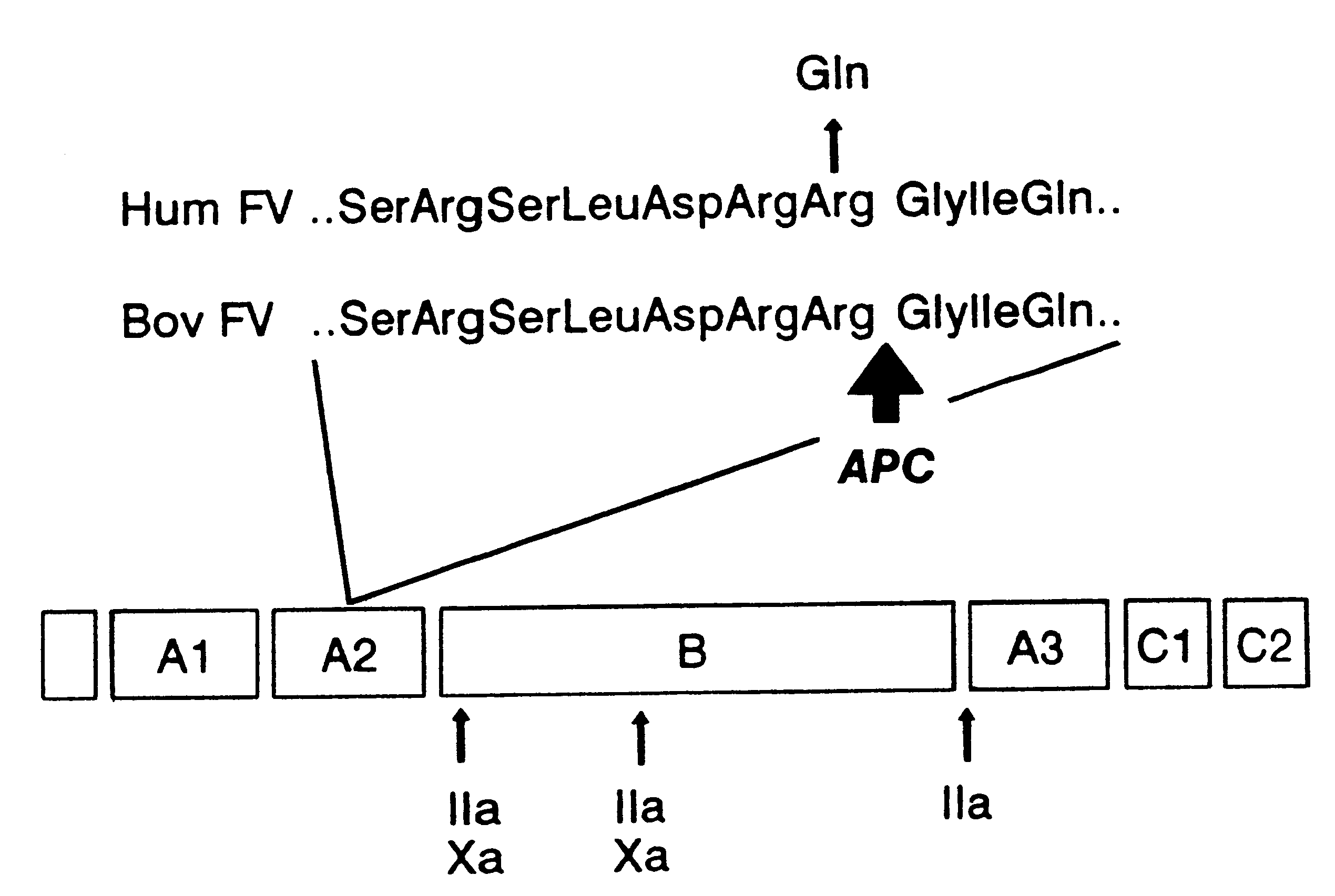
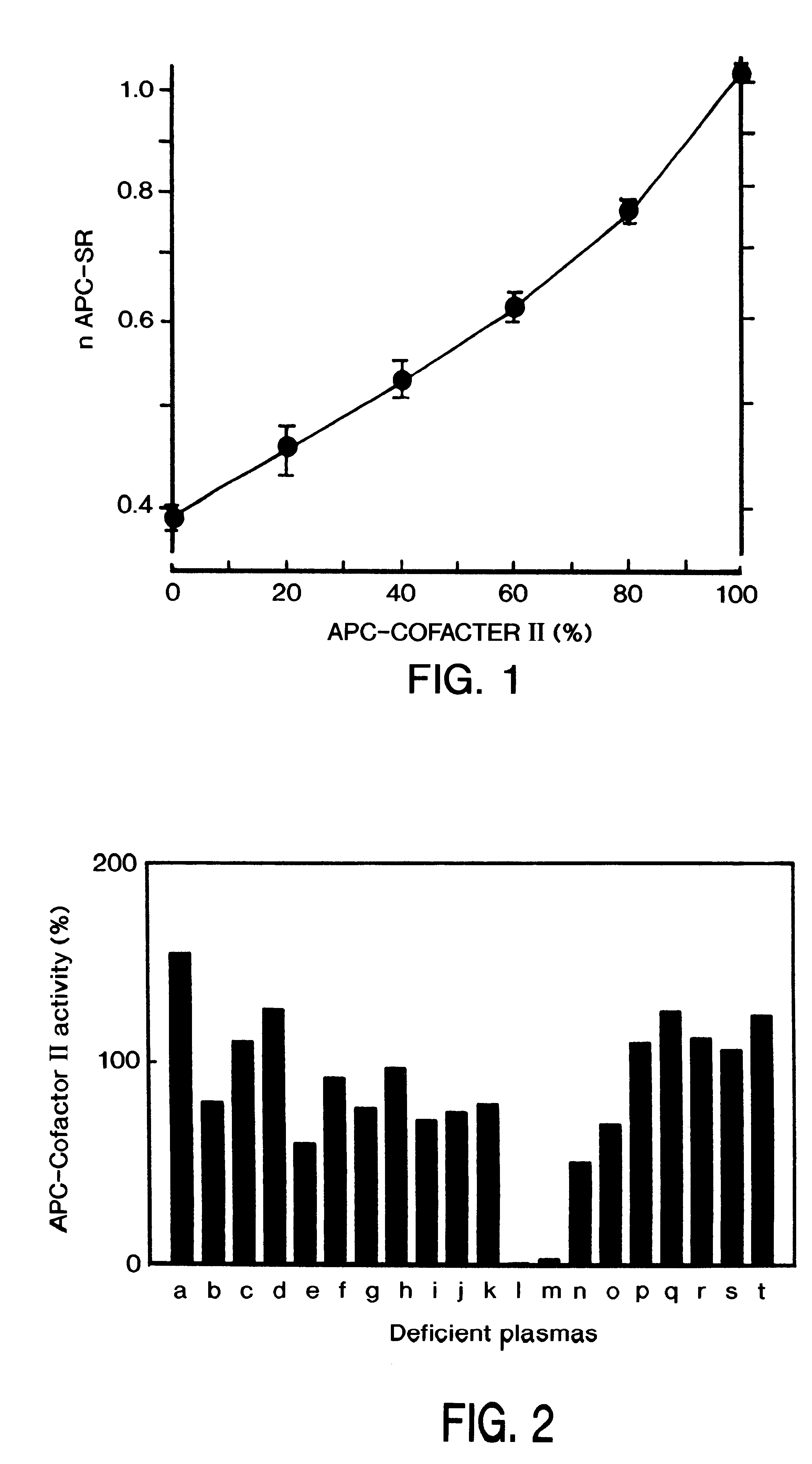
Method for screening for the presence of a genetic defect associated with thrombosis and / or poor anticoagulant response to activated protein C (APC). The method is directed at detecting one or more mutations at one or more of the cleavage and / or binding sites for APC of Factor V and / or Factor Va or at Factor VIII and / or Factor VIIIa at either nucleic acid or protein level or both.
Owner:AKZO NOBEL NV
Protein C variants
InactiveUS20050059132A1Improve propertiesHigh anticoagulant activityPeptide/protein ingredientsMammal material medical ingredientsProtein activationWild type
The present invention is concerned with a variant blood coagulation component, which is substantially homologous in amino acid sequences to a wild-type blood coagulation component capable of exhibiting anticoagulant activity in the protein C-anticoagulant system of blood and selected from protein C (PC) and activated protein C (APC), said variant component being capable of exhibiting an anticoagulant activity, that is enhanced in comparison with anticoagulant activity expressed by the corresponding wild-type blood coagulation component, said variant component differing from the respective wild-type component, in that it contains in comparison with said wild-type component at least one amino acid residue modification in it N-terminal amino acid residue sequence that constitutes the Gla-domain of protein C. The present invention is also concerned with methods to produce such variants based on DNA technology, with DNA segments intended for use in the said methods, and with use of said variants for therapeutic and diagnostic purposes.
Owner:DAHLBACK BJORN +1
Methods and compositions for activated protein c with reduced Anti-coagulant properties
InactiveUS20080058265A1Promote activationReduce bleeding riskNervous disorderPeptide/protein ingredientsProtein activationWild type
This invention relates to a novel form of protein C or activated protein C. More specifically, the invention is directed to a variant of protein C that is activated at a higher rate than wild-type or other variants and produces an activated protein C with reduced anticoagulant properties while retaining the protective anti-inflammatory and anti-apoptotic properties of wild-type activated protein C. This novel APC variant will be beneficial for treating inflammatory and apoptotic disorders with a reduced risk for bleeding.
Owner:SAINT LOUIS UNIVERSITY
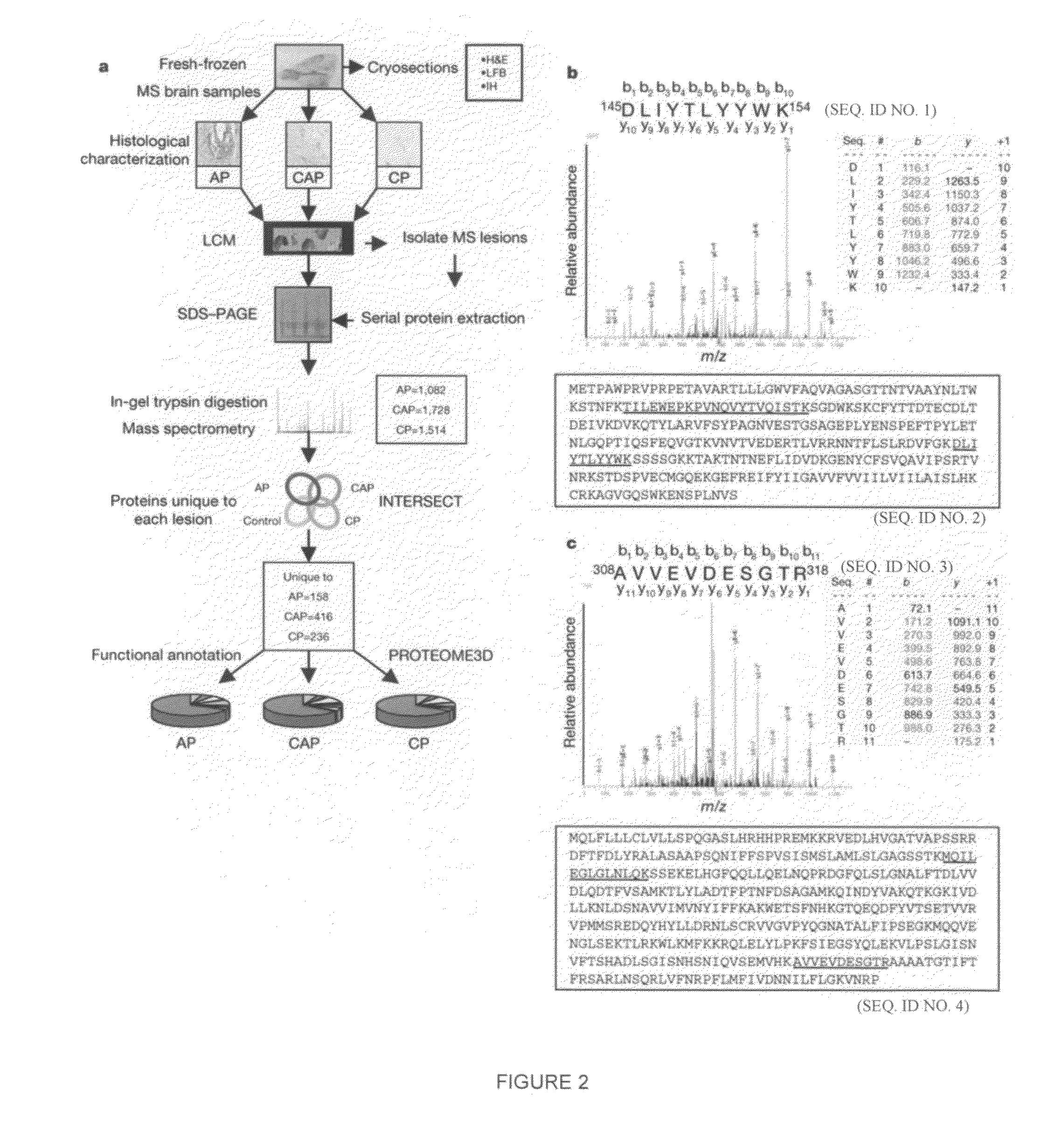
Proteomic analysis of active multiple sclerosis lesions
InactiveUS20090208481A1Improve diseaseSignificant clinical effectPeptide/protein ingredientsAntipyreticProtein activationChronic Active
The invention provides methods for treating demyelinating inflammatory diseases by administering to the subject an effective amount of an agent that provides activated protein C activity, where the dose is effective to reduce the adverse clinical indicia of the disease. In some embodiments, the patient being treating is of the chronic active plaque type.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Protein C or activated protein C-like molecules
InactiveUS20050095668A1Improve stabilityPeptide/protein ingredientsMammal material medical ingredientsHalf-lifeIn vivo
The present invention relates to novel conjugates between polypeptide variants of protein C and a non-polypeptide moiety, such as PEG or sugar moieties. In particular, the present invention provides novel protein C conjugates having an increased resistance to inactivation by e.g. human plasma and α1-antitrypsin. Consequently, such conjugates have an increased in vivo half-life. Preferred examples include protein C conjugates, wherein at least one additional in vivo N-glycosylation site has been introduced. The conjugates of the invention are useful for treating a variety of diseases, including septic shock.
Owner:MAXYGEN +1
Method for screening for the presence of genetic defect associated with thrombosis and/or poor anticoagulant response to activated protein C
InactiveUS6558913B1Immunoglobulins against blood coagulation factorsSugar derivativesBinding siteProtein activation
Method for screening for the presence of a genetic defect associated with thrombosis and / or poor anticoagulant response to activated protein C (APC). The method is directed at detecting one or more mutations at one or more of the cleavage and / or binding sites for APC of Factor V and / or Factor Va or at Factor VIII and / or Factor VIIIa at either nucleic acid or protein level or both.
Owner:AKZO NOBEL NV
Chemiluminescence immune detection reagent kit for detecting Clenbuterol
InactiveCN101413951AGuaranteed long-term effectivenessReduce usageChemiluminescene/bioluminescenceCross-linkChemiluminescent immunoassay
The invention provides a chemiluminescent immunoassay kit for detecting clenbuterol, and the kit belongs to the field of immunoassay. The kit is composed of a non-transparent white enzyme-labeled plate which is coated by a clenbuterol-carrier protein cross-linked complex, a clenbuterol standard product, a clenbuterol-peroxidase-labeled antibody, luminescent substrate solution and concentrated buffer solution. The clenbuterol-carrier protein cross-linked complex is obtained by coupling the clenbuterol and a carrier protein through the mixed-anhydride method or the direct protein activation method, and the concentrated buffer solution contains Tween-20 buffer solution. The kit has the advantages of rapidness, simpleness, high sensitivity, low detection cost, good repeatability, high throughput, and the like, and the kit can be used in the detection of residual clenbuterol in animal urine, blood, tissues, visceral organs and other samples.
Owner:SHANGHAI JIAO TONG UNIV
Activated protein C variants with normal cytoprotective activity but reduced anticoagulant activity
ActiveUS20050037964A1Reduced anticoagulant activityReduce bleeding riskAntibacterial agentsNervous disorderReperfusion injuryApoptosis
Variants (mutants) of recombinant activated protein C (APC) or recombinant protein C (prodrug, capable of being converted to APC) that have substantial reductions in anticoagulant activity but that retain normal levels of anti-apoptotic activity are provided. Two examples of such recombinant APC mutants are KKK191-193AAA-APC and RR229 / 230M-APC. APC variants and prodrugs of the invention have the desirable property of being cytoprotective (anti-apoptotic effects), while having significantly reduced risk of bleeding. The invention also provides a method of using the APC variants or prodrugs of the invention to treat subjects who will benefit from APC's cytoprotective activities that are independent of APC's anticoagulant activity. These subjects include patients at risk of damage to blood vessels or tissue in various organs caused, at least in part, by apoptosis. At risk patients include, for example, those suffering (severe) sepsis, ischemia / reperfusion injury, ischemic stroke, acute myocardial infarction, acute or chronic neurodegenerative diseases, or those undergoing organ transplantation or chemotherapy, among other conditions. Methods of screening for variants of recombinant protein C or APC that are useful in accordance with the invention are also provided.
Owner:THE SCRIPPS RES INST
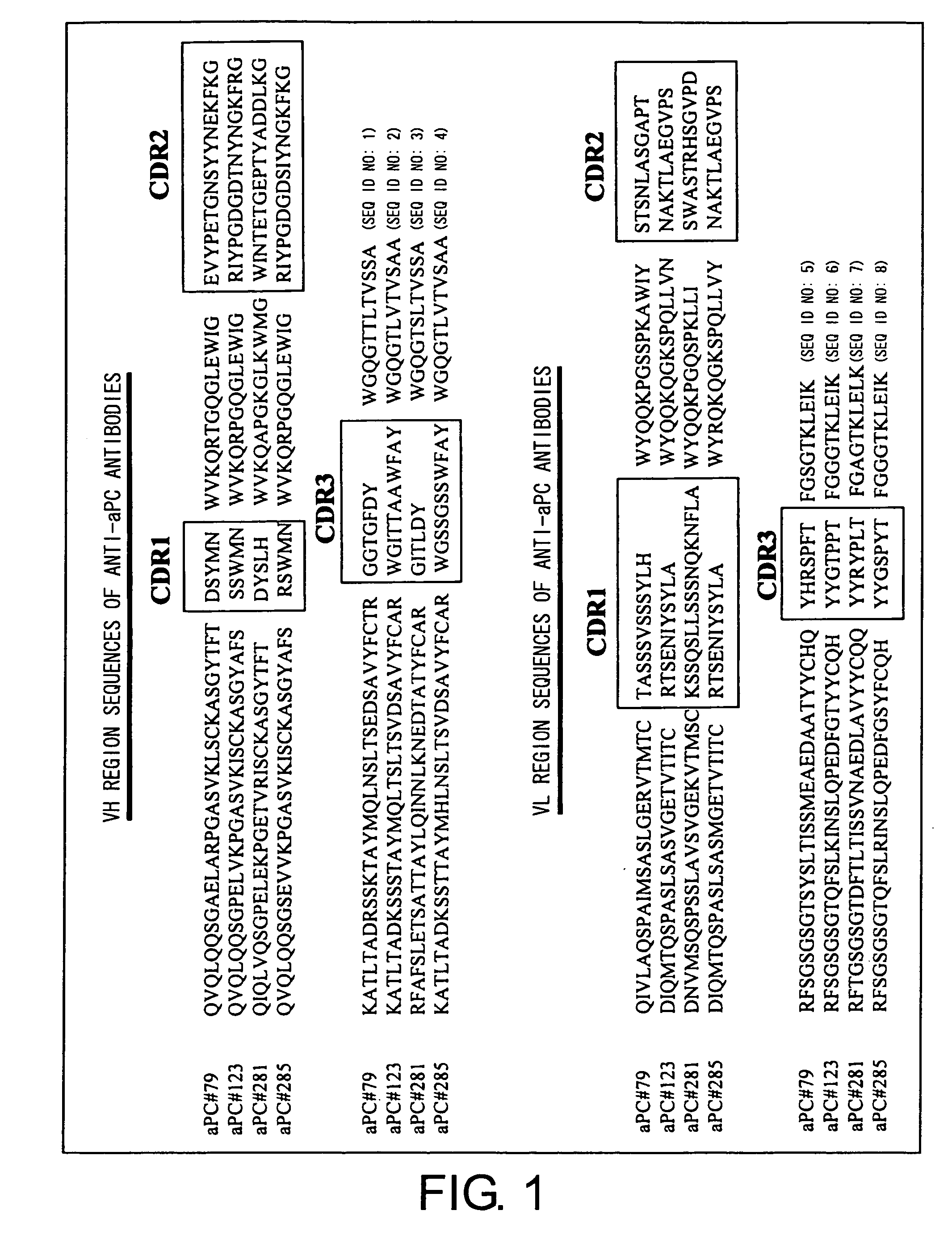
Monoclonal antibodies against activated protein C
ActiveUS8153766B2Immunoglobulins against blood coagulation factorsAntibacterial agentsProtein activationMonoclonal antibody
The present invention provides monoclonal antibodies that selectively bind to and inhibit activated protein C without binding to or inhibiting unactivated protein C. Other antibodies inhibit both activated protein C and activation of unactivated protein C. Methods of treatment employing these antibodies are described herein as are methods of screening for and detecting these antibodies.
Owner:OKLAHOMA MEDICAL RES FOUND
Neuroprotective, antithrombotic and anti-inflammatory uses of activated protein C (APC)
InactiveUS7074402B2Reduce inflammationReduce neuroinflammationBiocideNervous disorderRisk strokeDisease cause
The present invention provides methods for treating subjects having or at risk of having a neuropathological disorder or brain inflammatory diseases with and without vascular involvement, and systemic inflammatory vascular disease by administering a therapeutically effective amount of Activated Protein C (APC) to the subject. Brain disorders and brain inflammatory vascular diseases that can be treated by the invention method include all neurodegenerative diseases with different types of neuronal dysfunction, including stroke, Alzheimer's disease, Parkinson's disease, Huntington disease, neuroimmunological disorders such as multiple scelrosis and Gullian-Barre, encephalitis, meningitis, as well as other peripheral vascular diseases, such as diabetes, hypertension, artheriosclerosis. Also included are methods of treatment using APC in combination with a co-factor, such as Protein S.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Chemiluminescence immune detection reagent kit for detecting ractopamine
InactiveCN101413952AGuaranteed long-term effectivenessReduce usageChemiluminescene/bioluminescenceCross-linkChemiluminescent immunoassay
The invention provides a chemiluminescent immunoassay kit for detecting ractopamine, and the kit belongs to the field of immunoassay. The kit is composed of a non-transparent white enzyme-labeled plate which is coated by a ractopamine-carrier protein cross-linked complex, a ractopamine standard product, a ractopamine-peroxidase-labeled antibody, luminescent substrate solution and concentrated buffer solution. The ractopamine-carrier protein cross-linked complex is obtained by coupling the ractopamine and a carrier protein through the mixed-anhydride method or the direct protein activation method, and the concentrated buffer solution contains Tween-20 buffer solution. The kit has the advantages of rapidness, simpleness, high sensitivity, low detection cost, good repeatability, high throughput and the like, and the kit can be used for detecting the residual ractopamine in animal urine, blood, tissues, visceral organs and other samples.
Owner:SHANGHAI JIAO TONG UNIV
Compositions and methods comprising protein activated receptor antagonists
InactiveUS20060142203A1Minor side effectsAntibacterial agentsOrganic active ingredientsReceptor activationBiological activation
Compositions and methods comprising protein activated receptor antagonists are provided More particularly, the present invention relates to the use of proteins, peptides and biomolecules that bind to protein activated receptor 2, and inhibit the processes associated with the activation of that receptor. More specifically, the present invention provides novel compositions and methods for the treatment of disorders and diseases such as those associated with abnormal cellular proliferation, angiogenesis, inflammation and cancer.
Owner:ENTRE MED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com