Antibody capable of neutralizing substance having activity alternative to function of coagulation factor VIII (FVIII)
一种凝固因子、双特异性抗体的技术,应用在抗凝血因子免疫球蛋白、引入外来遗传物质而修饰的细胞、抗体等方向,能够解决凝固时间缩短、不可准确地测量FVIII活性和FVIII抑制剂滴度、影响测量FVIII测定系统等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0210] [Example 1] Production of Antibodies against Anti-FIXa / FX Bispecific Antibodies and Sequence Determination of Variable Regions
[0211] An attempt was made to generate an antibody against ACE910 (Q499-z121 / J327-z119 / L404-k) (bispecific antibody in which the H chain consisting of the amino acid sequence of SEQ ID NO: 9 is combined with the L chain of SEQ ID NO: 10, and the H chain consisting of the amino acid sequence of SEQ ID NO: 11 combined with the L chain of SEQ ID NO: 10), which is a bispecific antibody described in the patent document (WO 2012 / 067176). F(ab')2 consisting of individual Fabs on the anti-FIXa side and anti-FX side was generated using genetic recombination technology and pepsin digestion.
[0212] Mice and rats were immunized with anti-FIXa-F(ab')2 or anti-FX-F(ab')2. Cell fusion of cells obtained from spleens removed from mice or rats or from lymph nodes of rats with mouse myeloma cells was performed according to general methods to produce hybridoma...
Embodiment 2
[0213] [Example 2] Production of expression vectors for recombinant mouse antibody AQ8 and recombinant rat-rabbit chimeric antibody AJ540.
[0214]The recombinant mouse antibody AQ8 was prepared as follows: The variable region sequence of the AQ8 antibody obtained in Example 1 was combined with the known mouse IgG2b constant region sequence (heavy chain: EMBL Accession No. J00461; light chain: EMBL Accession No. V00807) combined to produce a full-length antibody gene, which is then inserted into an expression vector. Similarly, recombinant rat-rabbit chimeric antibody AJ540 was generated by combining known rabbit IgG (heavy chain: EMBL Accession No. L29172, light chain: EMBL Accession No. X00231) with the variable region of the AJ540 antibody. The resulting expression clone plasmids were introduced into HEK293 cells, large-scale culture and purification using protein A and gel filtration were performed, and recombinant mouse antibody AQ8 (rAQ8-mIgG2b) and recombinant rat-rabbi...
Embodiment 3
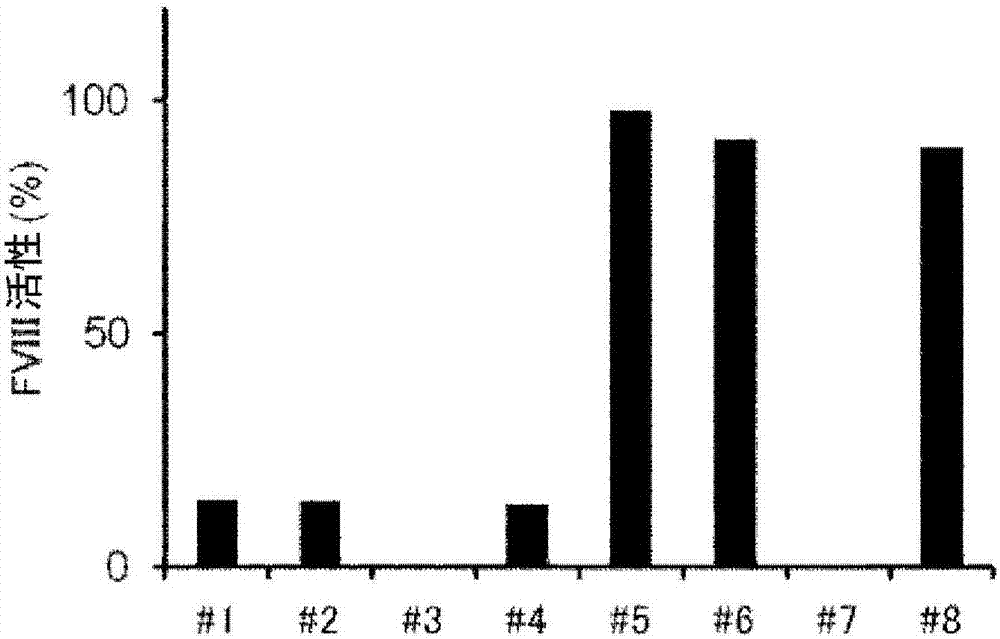
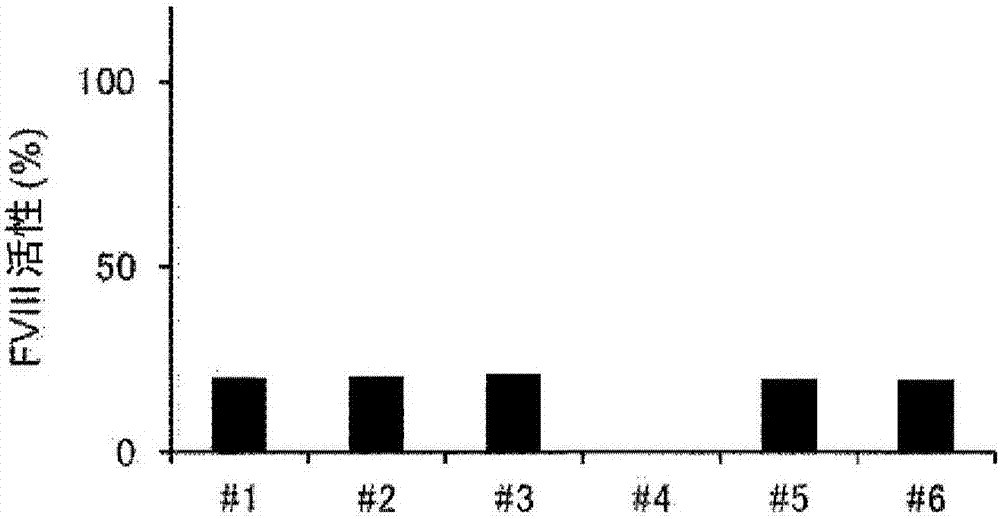
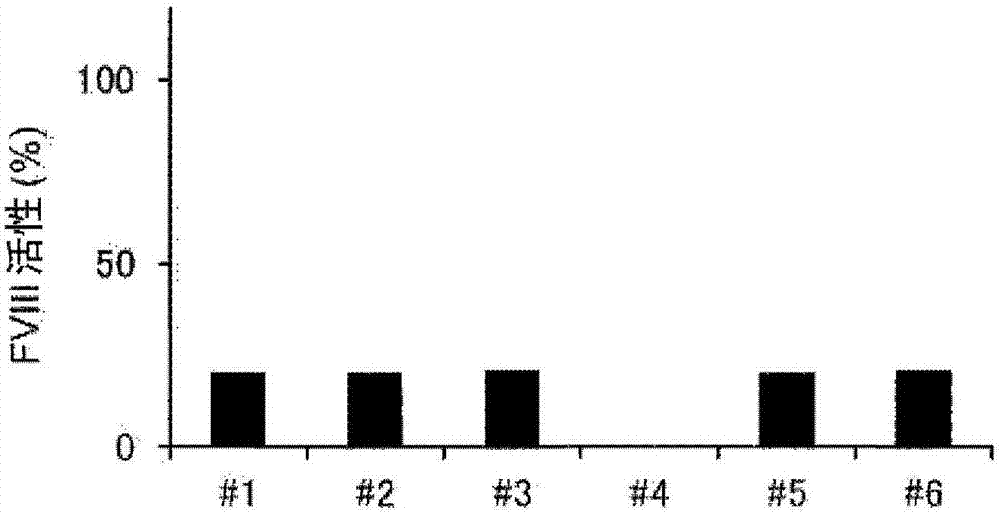
[0215] [Example 3] One-step coagulation assay under neutralization of anti-FIXa / FX bispecific antibody using rAQ8-mIgG2b and rAJ540-rbtIgG
[0216] To FVIII-deficient plasma (George King) containing 10 U / dL or 100 U / dL recombinant FVIII (Kogenate FS, Bayer Yakuhin, Ltd.), 0 μg / mL or 300 μg / mL of anti-FIXa / FX bispecific Antibody ACE910. In addition, each of the prepared plasma samples was divided into the following two groups to prepare measurement sample solutions: one group was diluted 10 times using imidazole buffer (Kyowa Medex); -RbtIgG was diluted 10-fold in imidazole buffer. The amount of rAQ8-mIgG2b and rAJ540-rbtIgG required to sufficiently neutralize ACE910 was added. Details of the combination are shown below.
[0217] [Table 1]
[0218]
[0219] Furthermore, in order to generate a calibration curve for converting clotting time into FVIII activity, 10-fold, 20-fold, 40-fold, 80-fold, and 160-fold dilutions were performed by using an imidazole buffer (the FVIII...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com