Severe hemophilia A disease-causing gene mutation detection kit
A technology for detecting kits and disease-causing genes, which is applied in the fields of biotechnology and biomedicine, can solve the problems of low cost requirements, cumbersome operation, and high cost, and achieve enhanced amplification specificity, improved amplification efficiency, and improved stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
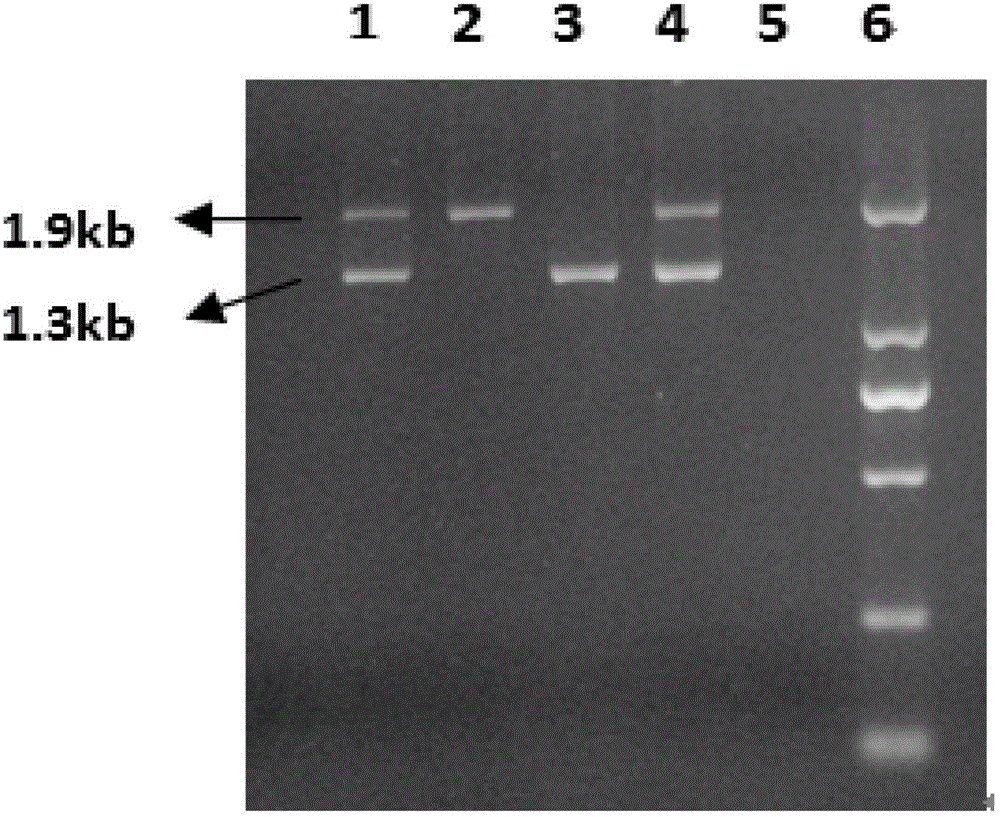
[0057] Embodiment 1 kit of the present invention forms
[0058] The characteristics of the kit of the present invention include Inv22PCR reaction solution, Inv1PCR reaction solution, Inv22 positive reference substance, Inv1 positive reference substance, and negative reference substance.
[0059] Inv22 PCR reaction includes Taq DNA polymerase, PCR buffer, Deaza-dGTP, dNTP, primers and Taq-Antibody (Ap);
[0060] Inv1PCR reaction solution includes Taq DNA polymerase, PCR buffer, dNTP, primers and Ap;
[0061] Inv22 positive reference product is a mixed plasmid containing all fragments of Inv22 PCR amplification products;
[0062] Inv1 positive reference product is a mixed plasmid containing all fragments of Inv1 PCR amplification products;
[0063] The composition of the negative reference is ddH2O;
[0064] 1. Primer sequence design
[0065] According to the reported FVIII gene inversion mechanism and sequence of severe hemophilia A, a large number of practical screenings w...
Embodiment 2
[0086] Embodiment 2 The use of kit of the present invention
[0087] 1. Storage conditions and validity period
[0088] Storage conditions: the kit should be stored below -18°C
[0089] Validity: 6 months
[0090] 2. Applicable instruments
[0091] PCR instrument (Heima 9600), ABI9700, etc.
[0092] 3. Sample requirements
[0093] The sample source of this kit is anticoagulated whole blood, and the anticoagulant used is sodium citrate or EDTA, and heparin cannot be used for anticoagulation.
[0094]Sample collection: Take 5mL of venous blood, put it into a tube containing anticoagulant, and mark the sample information such as the name and number of the sample.
[0095] Blood sample storage: Anticoagulated whole blood should be stored at room temperature for no more than 24 hours, stored at 2-8°C for no more than one month, stored in a refrigerator below -18°C for no more than two years, and stored in a refrigerator at -70°C for a long time. freeze-thaw.
[0096] Blood s...
Embodiment 3
[0117] Embodiment 3 kit of the present invention compares with prior art detection stability
[0118] The kit of the present invention solves the problems of reagent storage time, temperature tolerance and the like by adding a Taq DNA polymerase inhibitor. The Inv22 patent formulation of Liu and Sommer was selected as a comparison, and the patent number is US6355422B1. This patent uses long-distance PCR to detect hemophilia A intron 22 inversion mutation. The formula is a conventional PCR reaction solution, including Taq DNA polymerase, primers, dNTP, deaza-GTP and high concentration of DMSO.
[0119] In this test, according to the kit described in Example 1 of the present invention and the patented formula invented by Liu et al., three batches of products were successively trial-produced to detect the stability of Inv22. The batch numbers of the kit of the present invention are YN01, YN02 and YN03, and the batch numbers of the control reagents are LS01, LS02 and LS03.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com