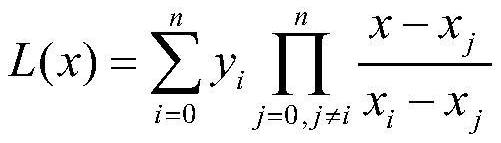Patents
Literature
45 results about "Patient screening" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
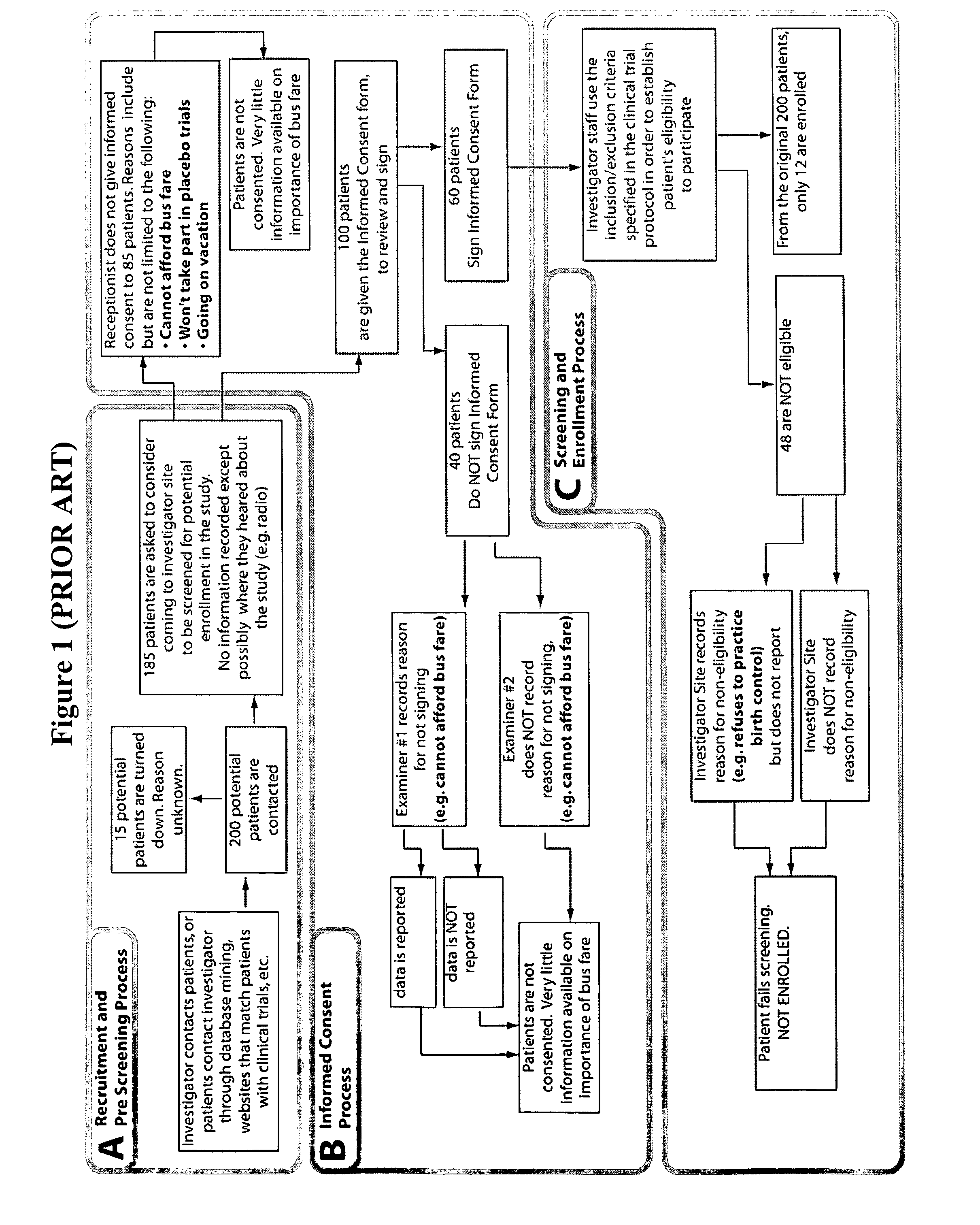
A screening test is a test provided to a patient in the absence of signs or symptoms based on the patient’s age, gender, medical history and family history according to medical guidelines. It is defined by the population on which the test is performed, not the results or findings of the test.
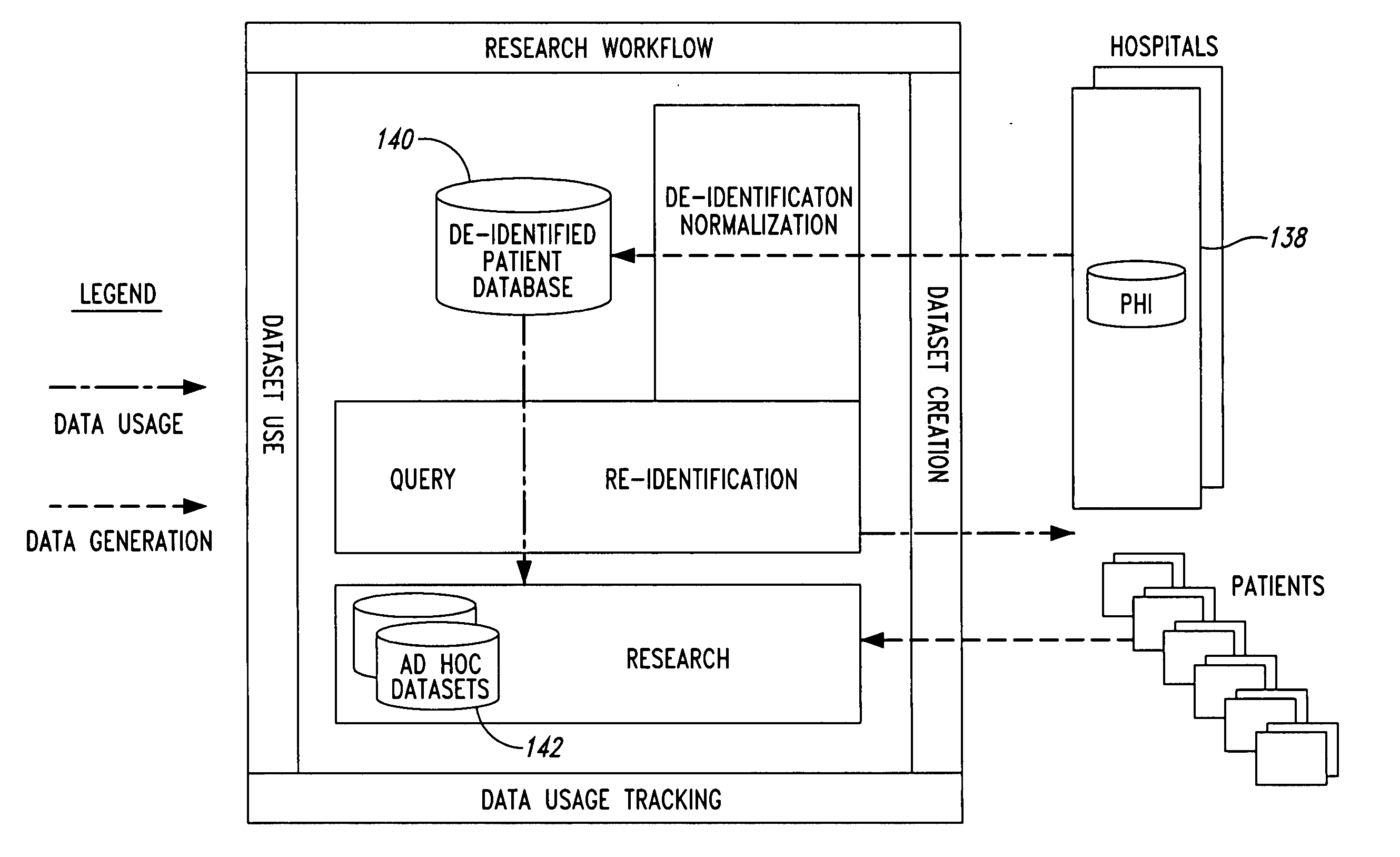
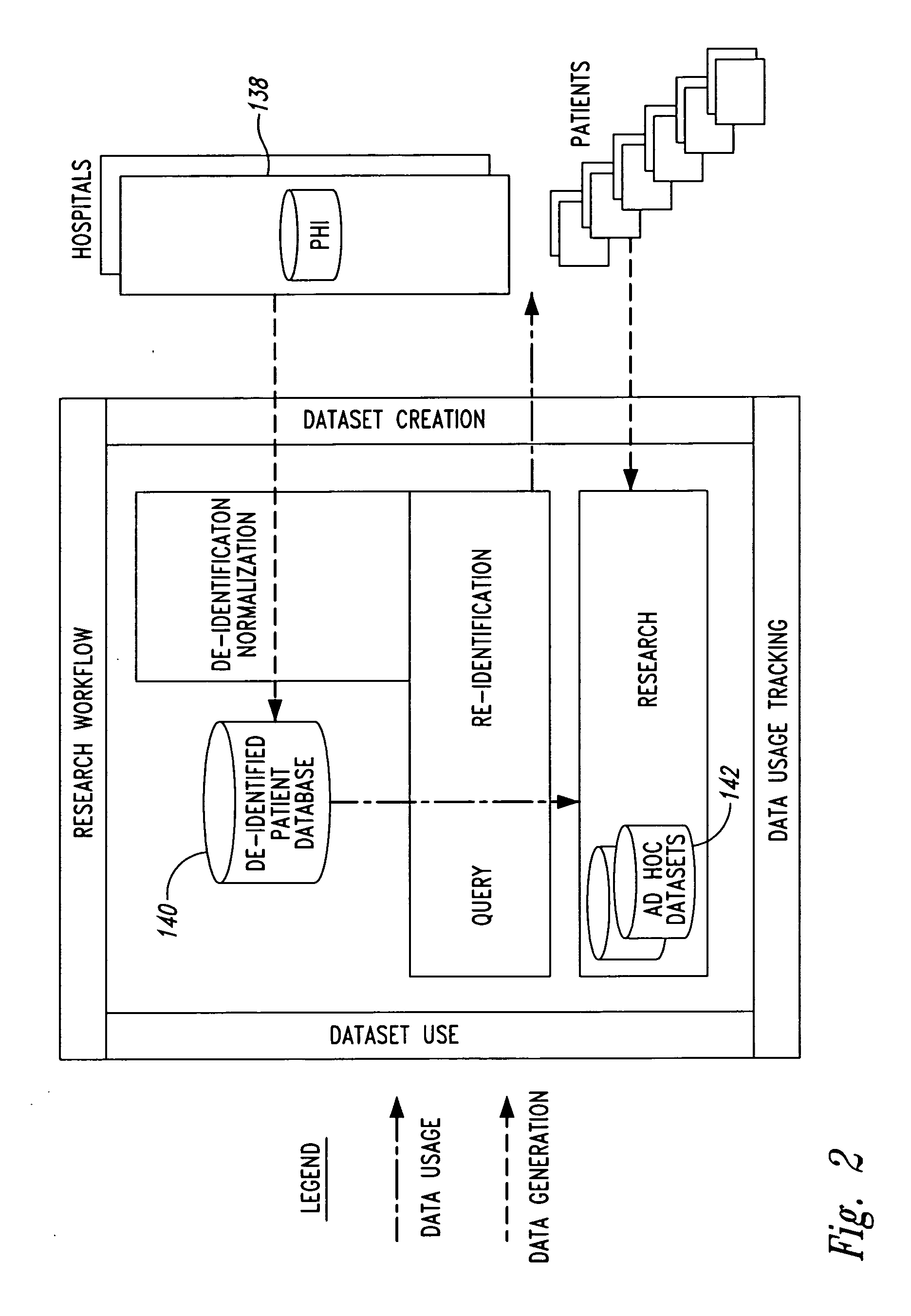
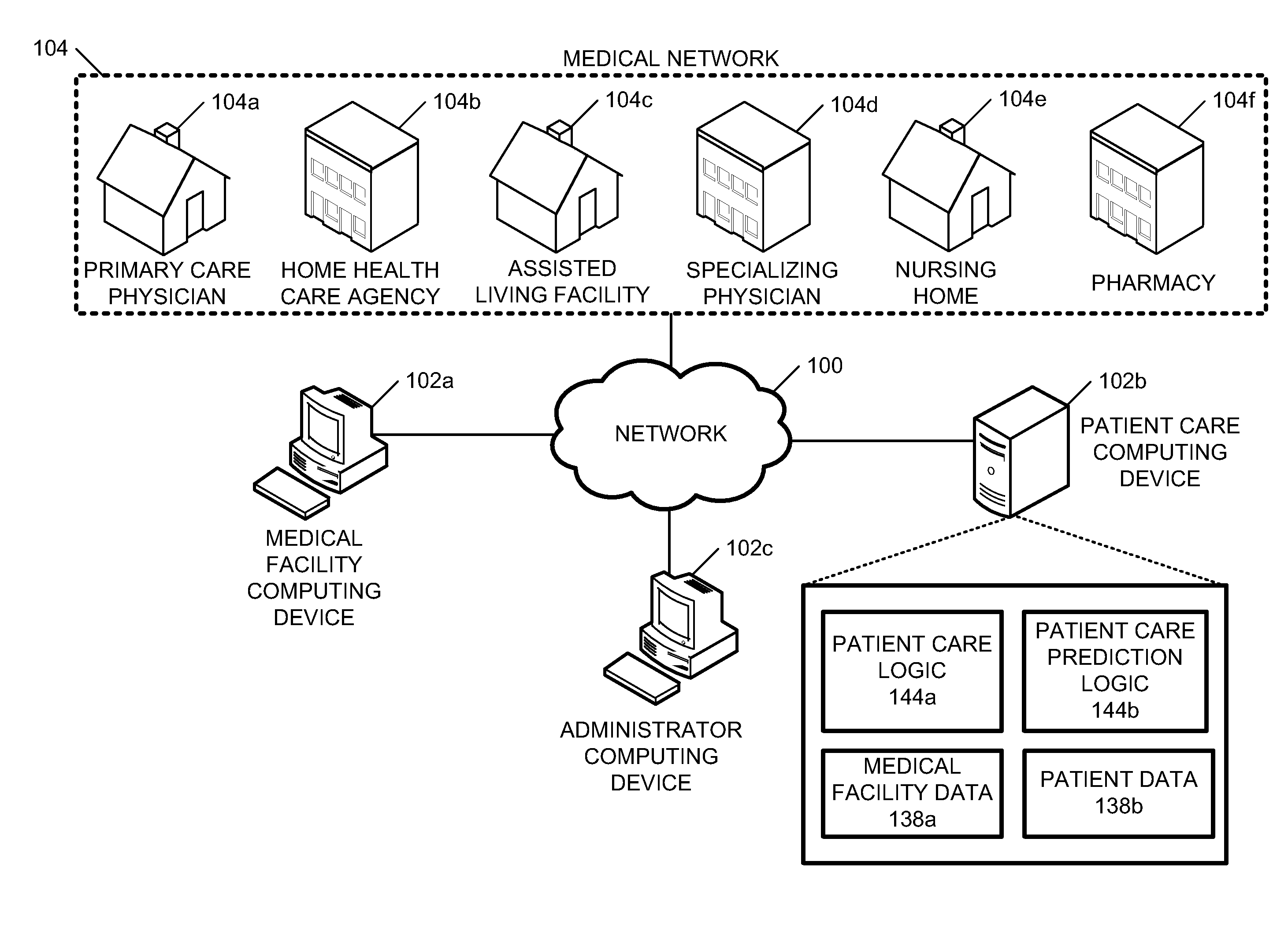
System and method for controlling access and use of patient medical data records
InactiveUS20050236474A1Prohibitive to printComputer security arrangementsPatient personal data managementConfidentialityPatient database
A system for processing patient health information (PHI) protects the confidentiality of PHI to achieve regulatory compliance. The PHI contains patient medical data and associated patient identification data. A de-identification agent extracts patient medical data and separates from all identification data to create de-identified patient data. A key is generated that allows subsequent reassociation of the patient medical data and the patient identification data. The de-identified patient data base may be queried for patient screening purposes. Patient queries are processed only if the study or patient screening has been authorized by appropriate authorities, such as an internal review board. Patients whose medical characteristics conform with the patient query are selected for possible use in a study. If re-identification of the selected patients is necessary, and authorized, the key may be used to provide the necessary reassociation. A data log records all access to patient data.
Owner:CONVERGENCE CT
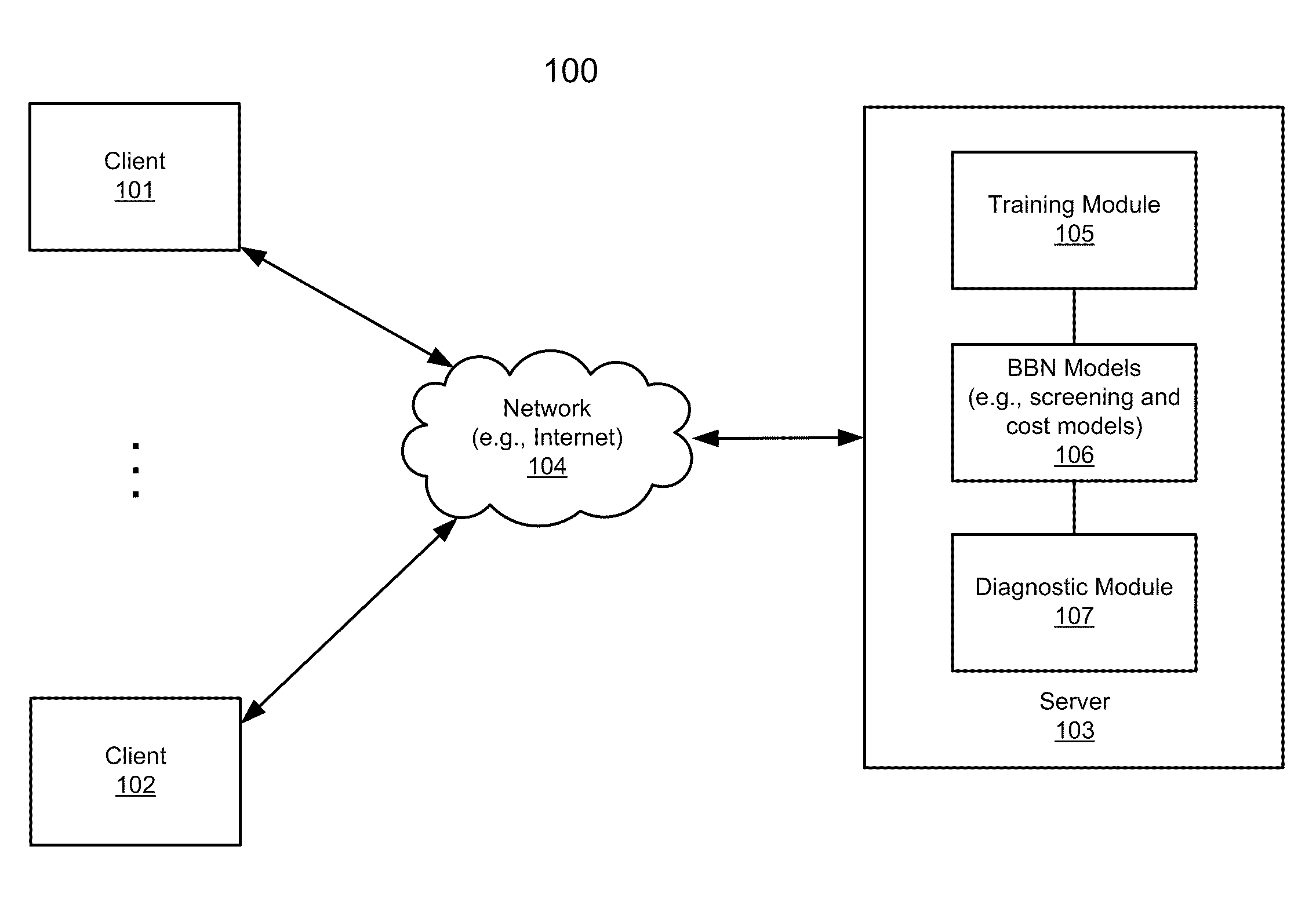
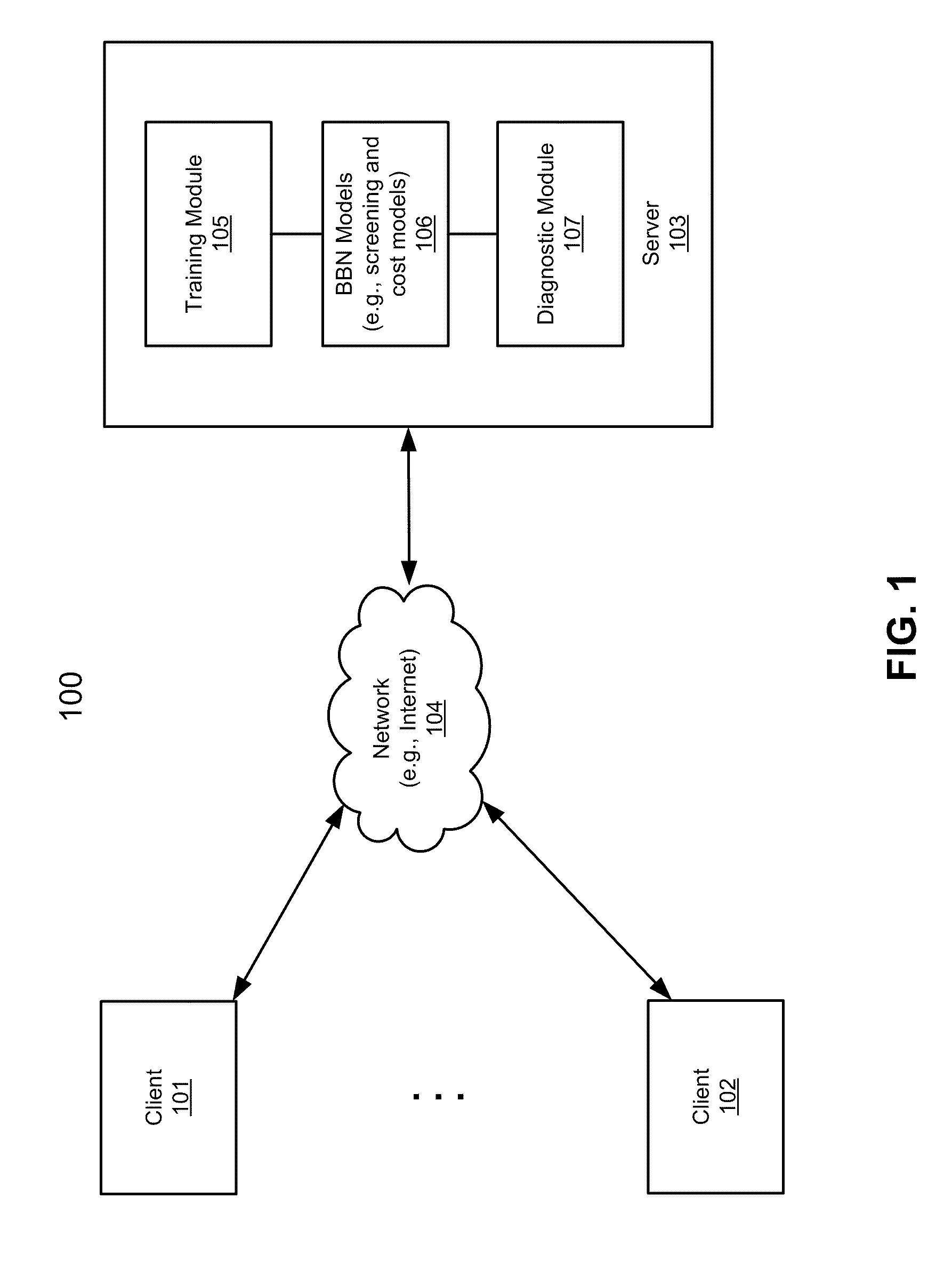
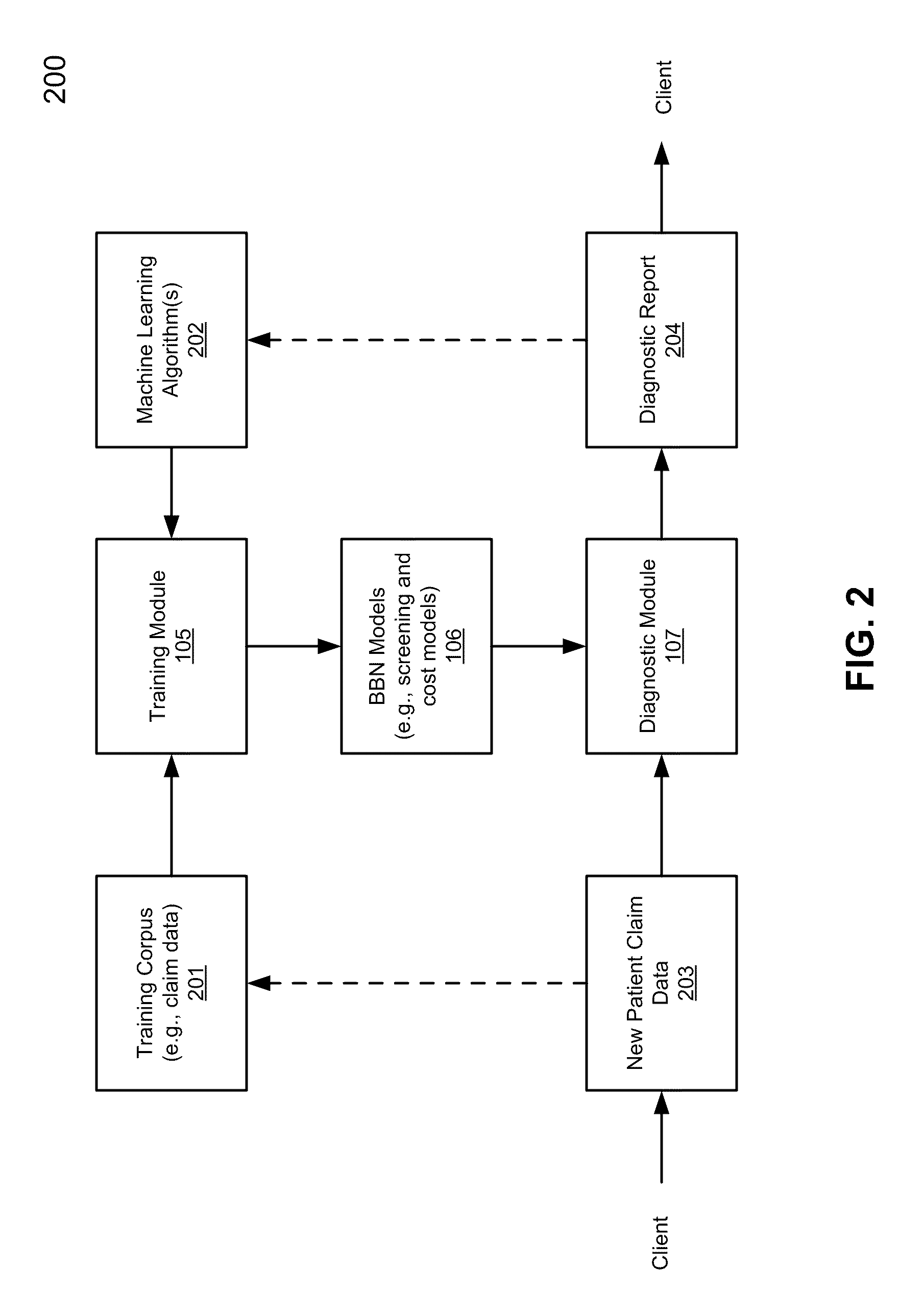
Application of bayesian networks to patient screening and treatment
According to one aspect of the invention, health insurance claim data for a first group of individuals is obtained to generate a training corpus, including a training set of claim data and a holdout set of claim data. The first group of individuals represents enrollees of one or more first health insurance plans and the health insurance claim data represents historic insurance claim information for each individual in the first group. A Bayesian belief network (BBN) model is created by training a BBN network based on the training set of claim data using predetermined machine learning algorithms. The BBN model is validated using the holdout set of claim data. The BBN model, when having been successfully validated, is configured to identify at least one of individuals with risk for a disorder and individuals with risk who are most likely to benefit from intervention and treatment for the disorder.
Owner:DECISIONQ CORP
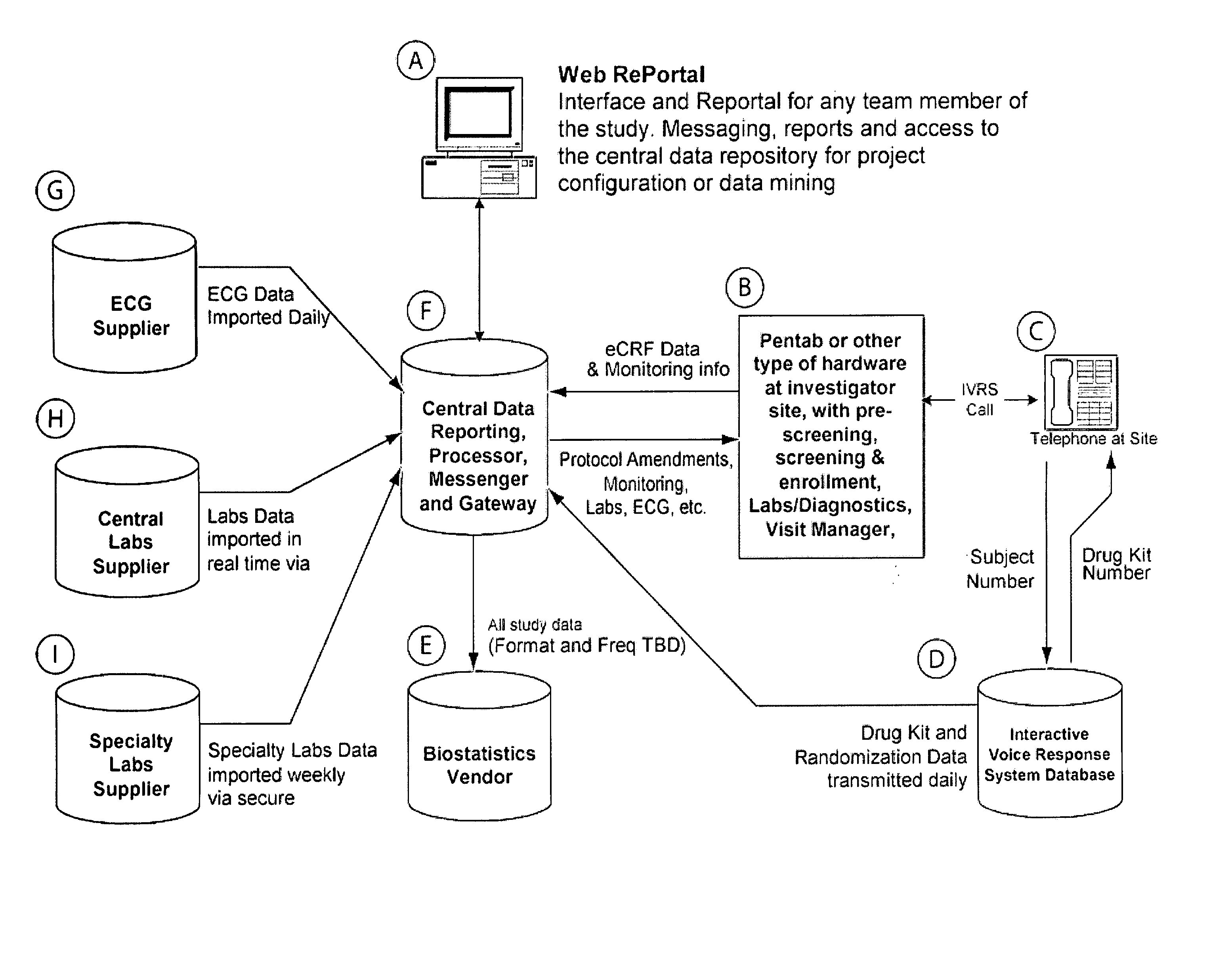
Method and apparatus for screening, enrollment and management of patients in clinical trials
InactiveUS20070067189A1Simple speedImproves of enrollment processData processing applicationsComputer-assisted medical data acquisitionDiagnostic testData center
A computer-implemented method of tracking patient data in a clinical trial is provided. The clinical trial has one or more investigative sites which perform patient screening and enrollment for the clinical trial, one or more diagnostic sites which perform analysis on one or more patient diagnostic tests ordered by an investigative site and generate analysis results, and a centralized data center in electronic communication with the one or more investigative sites and the one or more diagnostic sites. Each investigative site is provided with a user interface display screen for allowing a user at the investigative site to enter data regarding patients who have been screened for the clinical trial and patients who have been enrolled in the clinical trial. The data from each of the investigative sites is electronically communicated to the centralized data center. Also, the analysis results from each of the diagnostic sites are electronically communicated to the centralized data center. The centralized data center consolidates the data and analysis results from each of the sites and provides one or more status reports regarding the patients for whom data and analysis results were received from the one or more investigative sites and the one or more diagnostic sites.
Owner:NUMODA TECH
Patient data collection system and methods
InactiveUS7163516B1Easy to measurePerson identificationAudiometeringHearing acuityMeasurement device
A system for collecting patient screening data is disclosed. The system includes force measuring apparatus for measuring weight and for measuring balance forces, and stimuli delivery apparatus for delivering first stimuli designed to evaluate visual acuity, for delivering second stimuli designed to evaluate hearing acuity, and for accepting patient responses to the first stimuli and the second stimuli. A processing unit is associated with and manages the operation of the force measuring apparatus and the stimuli delivery apparatus for collecting weight and balance force measurements from the force measuring apparatus and patient responses from the stimuli delivery apparatus.
Owner:PAGNACCO GUIDO +2
Method of Risk Management for Patients Undergoing Natalizumab Treatment
Progressive multifocal leukoencephalopathy (PML) has been identified in patients taking natalizumab (NMAB) for the treatment of multiple sclerosis (MS). This patent application provides a novel method of patient screening and monitoring intended to decrease the risk of PML and other opportunistic central nervous system (CNS) diseases in patients undergoing MS therapy with NMAB, and proposes a novel method of screening and monitoring intended to decrease the risk of opportunistic disease processes of the CNS during the treatment of other medical disorders with NMAB.
Owner:SEEDLINGS LIFE SCI VENTURES
Cystic fibrosis gene
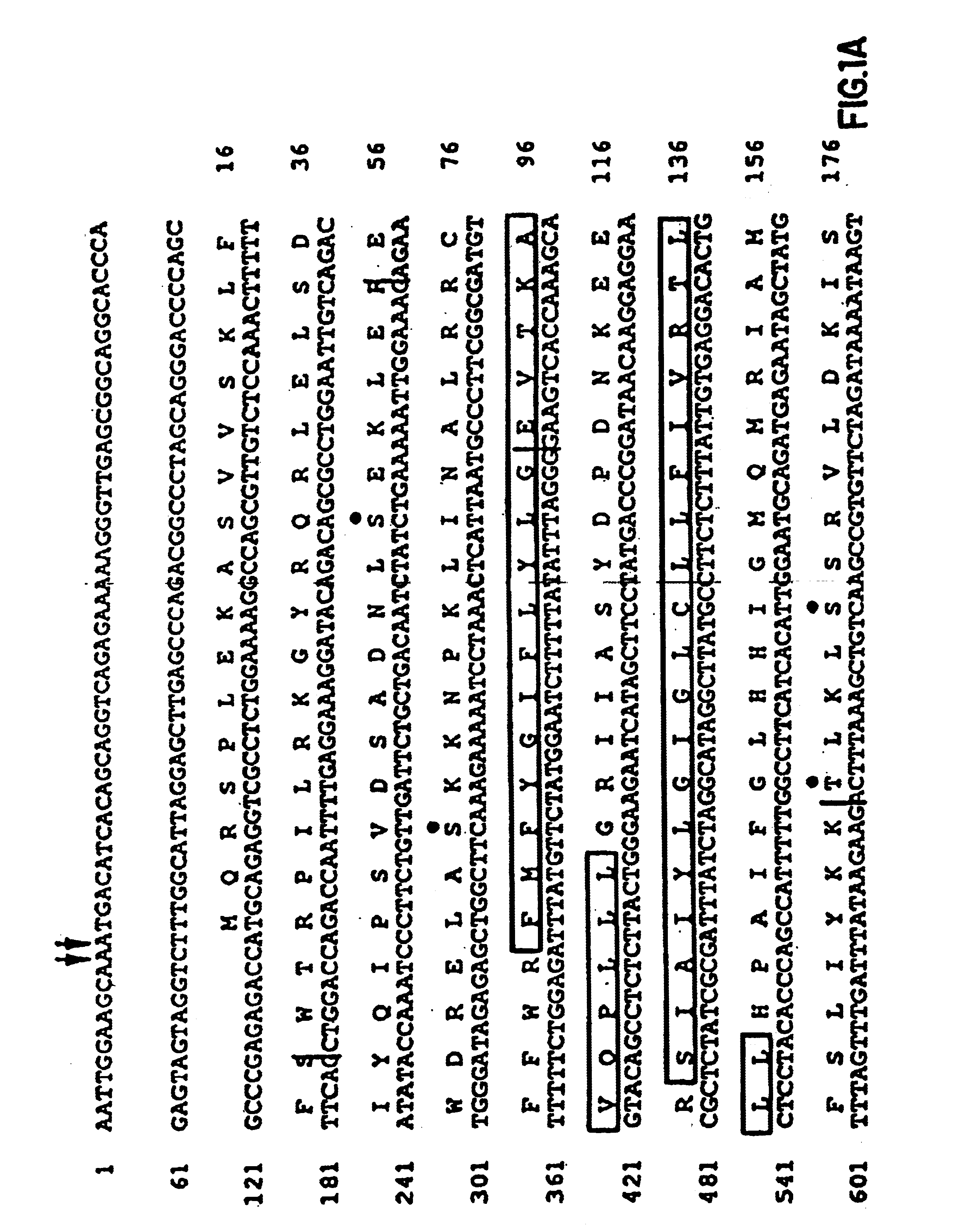
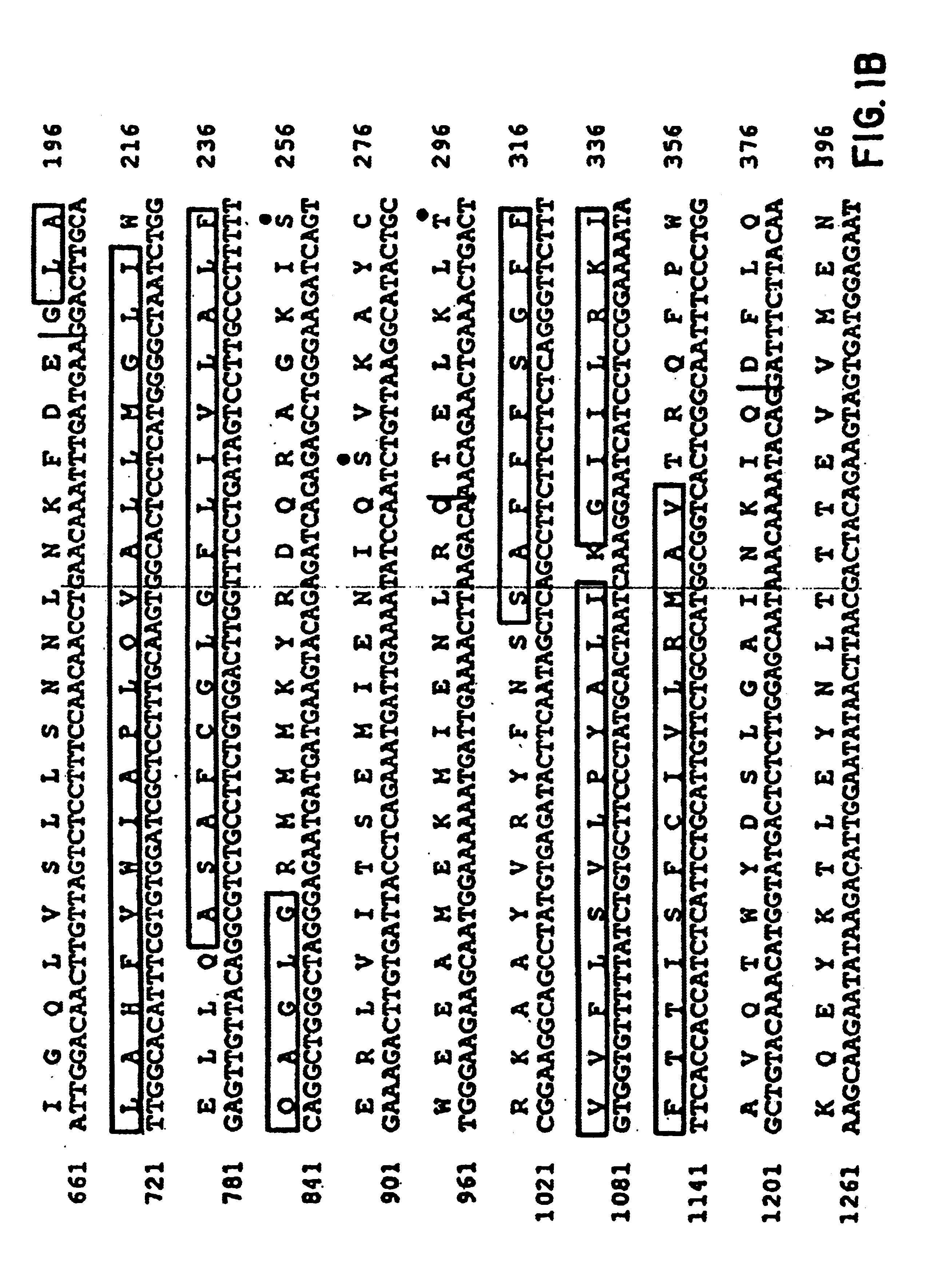
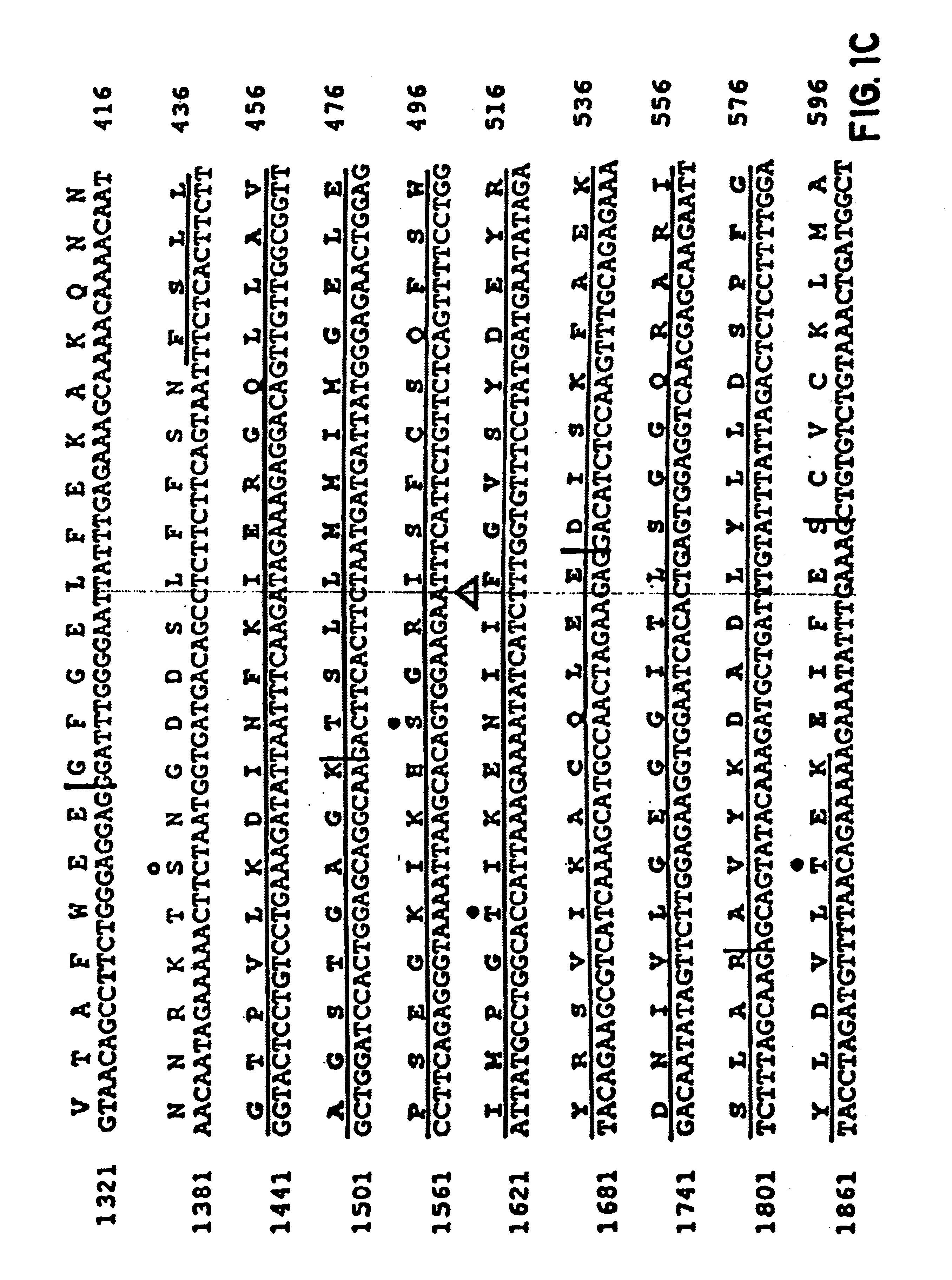
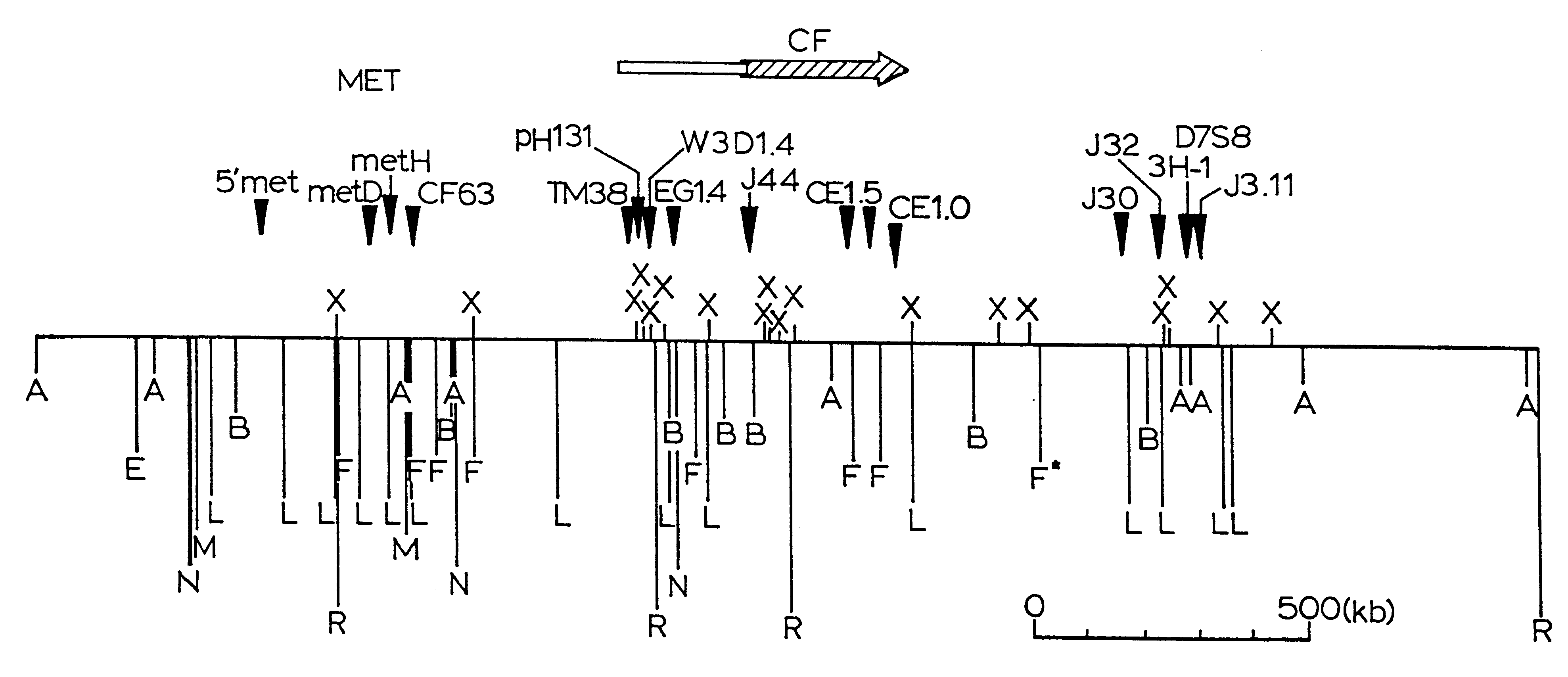
InactiveUS6902907B1Increase productionBacteriaGenetic material ingredientsGene productCystic fibrosis gene
The cystic fibrosis gene and its gene product are described for both the normal and mutant forms. The genetic and protein information is used in developing DNA diagnosis, protein diagnosis, carrier and patient screening, drug and gene therapy, cloning of the gene and manufacture of the protein, and development of cystic fibrosis affected animals.
Owner:HSC RES & DEV LLP +1
Cystic fibrosis gene
InactiveUS6984487B1Sugar derivativesMicrobiological testing/measurementCystic fibrosis geneGene product
The cystic fibrosis gene and its gene product are described for both the normal and mutant forms. The genetic and protein information is used in developing DNA diagnosis, protein diagnosis, carrier and patient screening, drug and gene therapy, cloning of the gene and manufacture of the protein, and development of cystic fibrosis affected animals.
Owner:HSC RES & DEV LLP +1
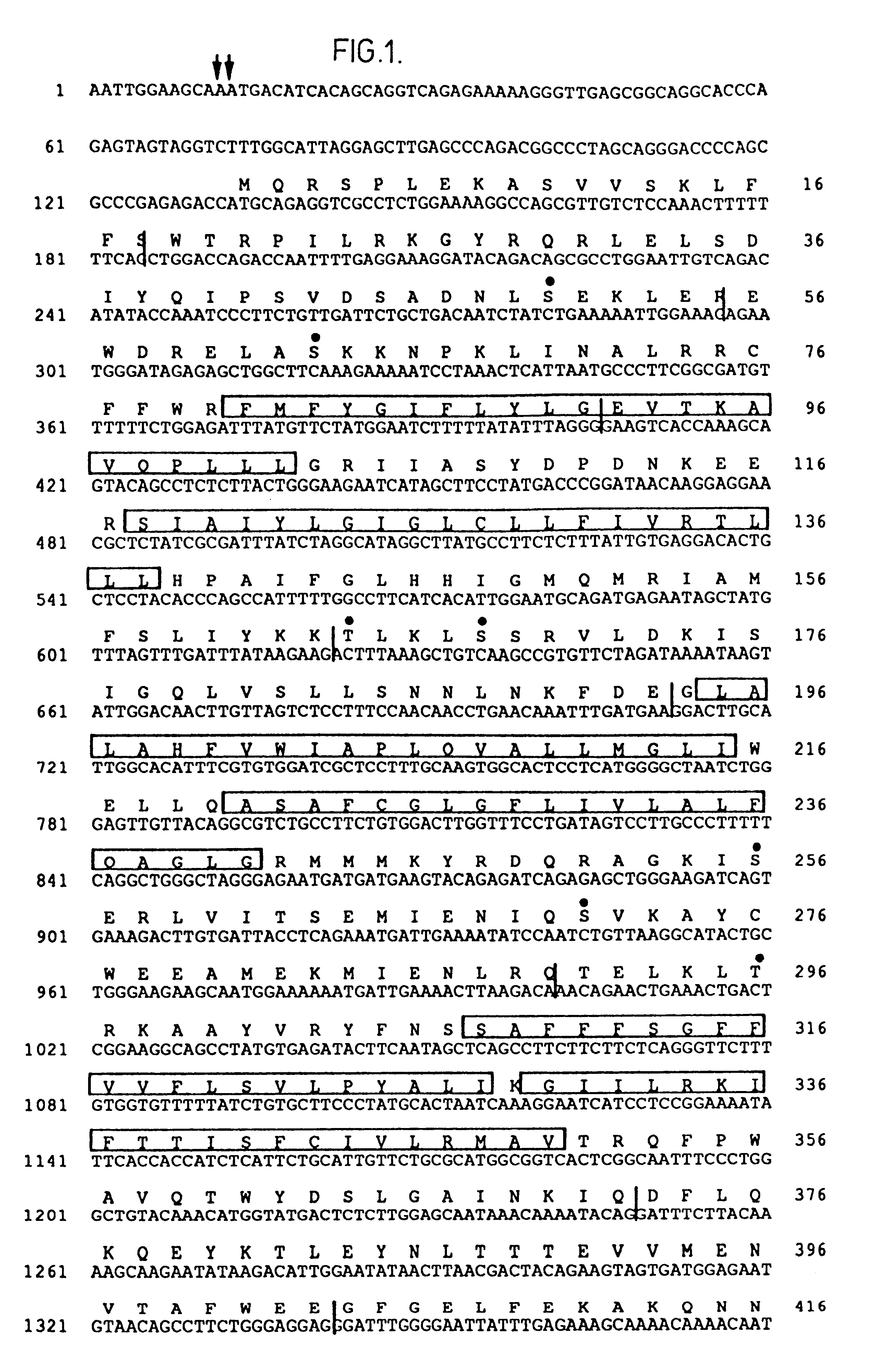
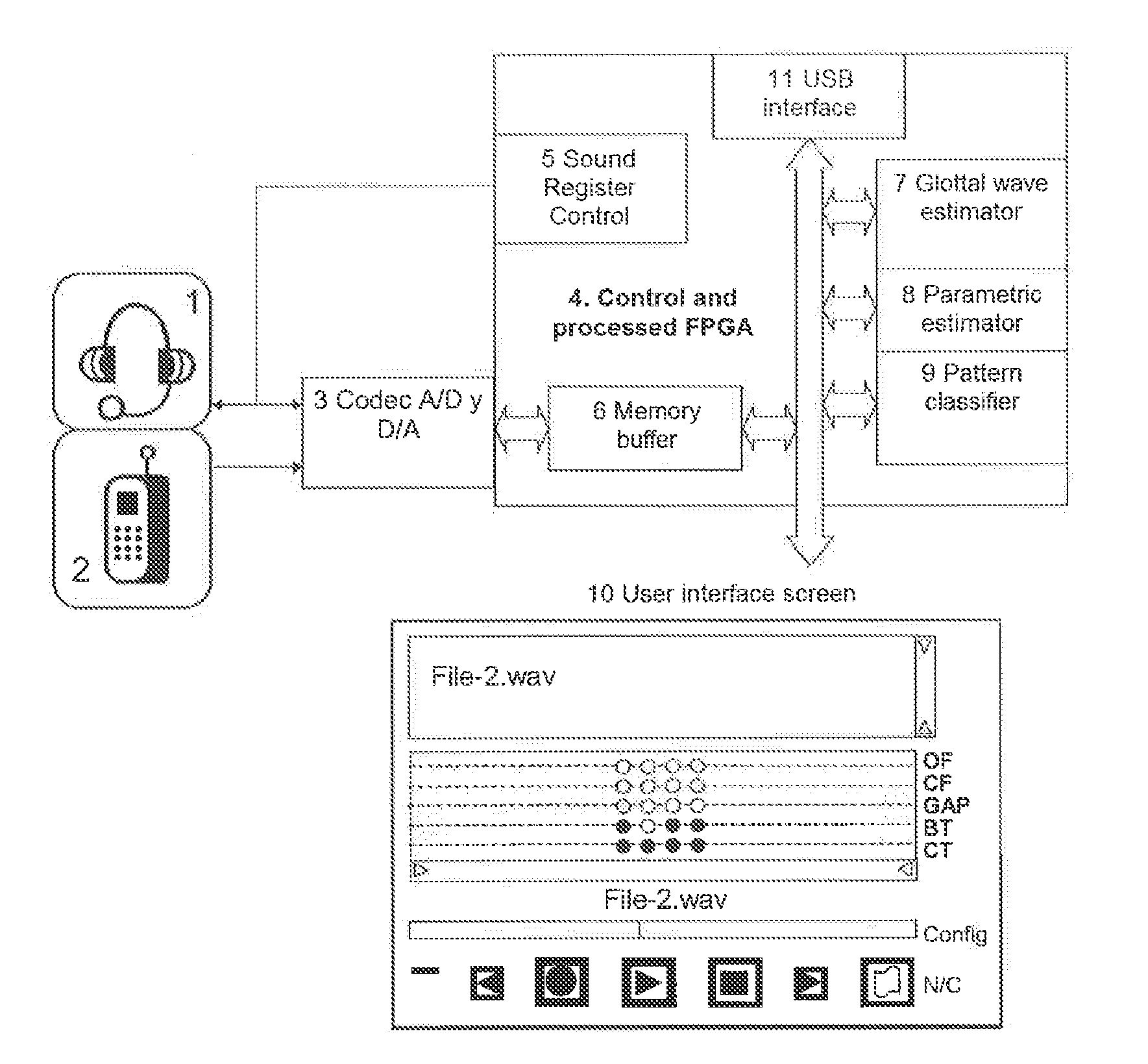
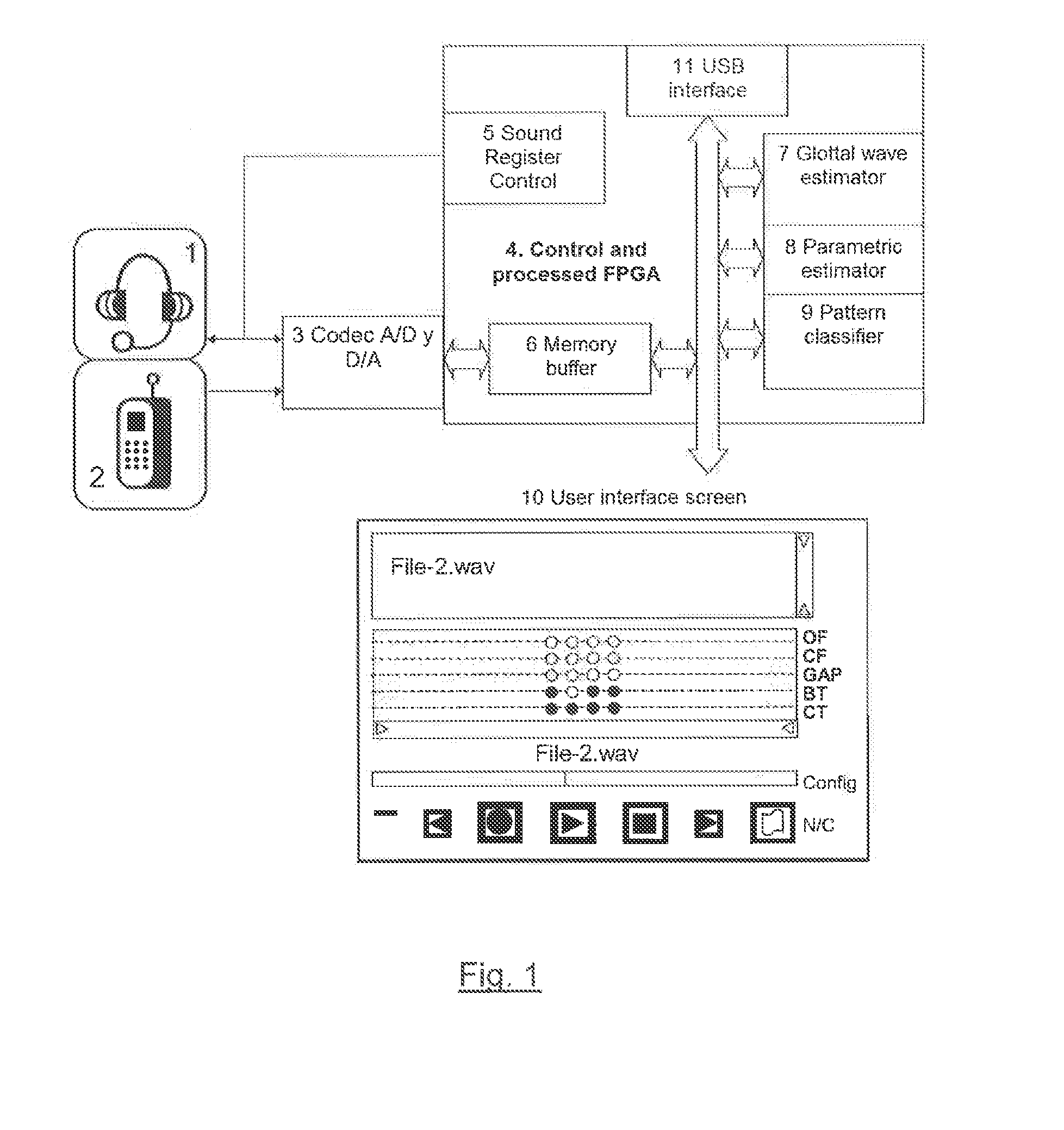
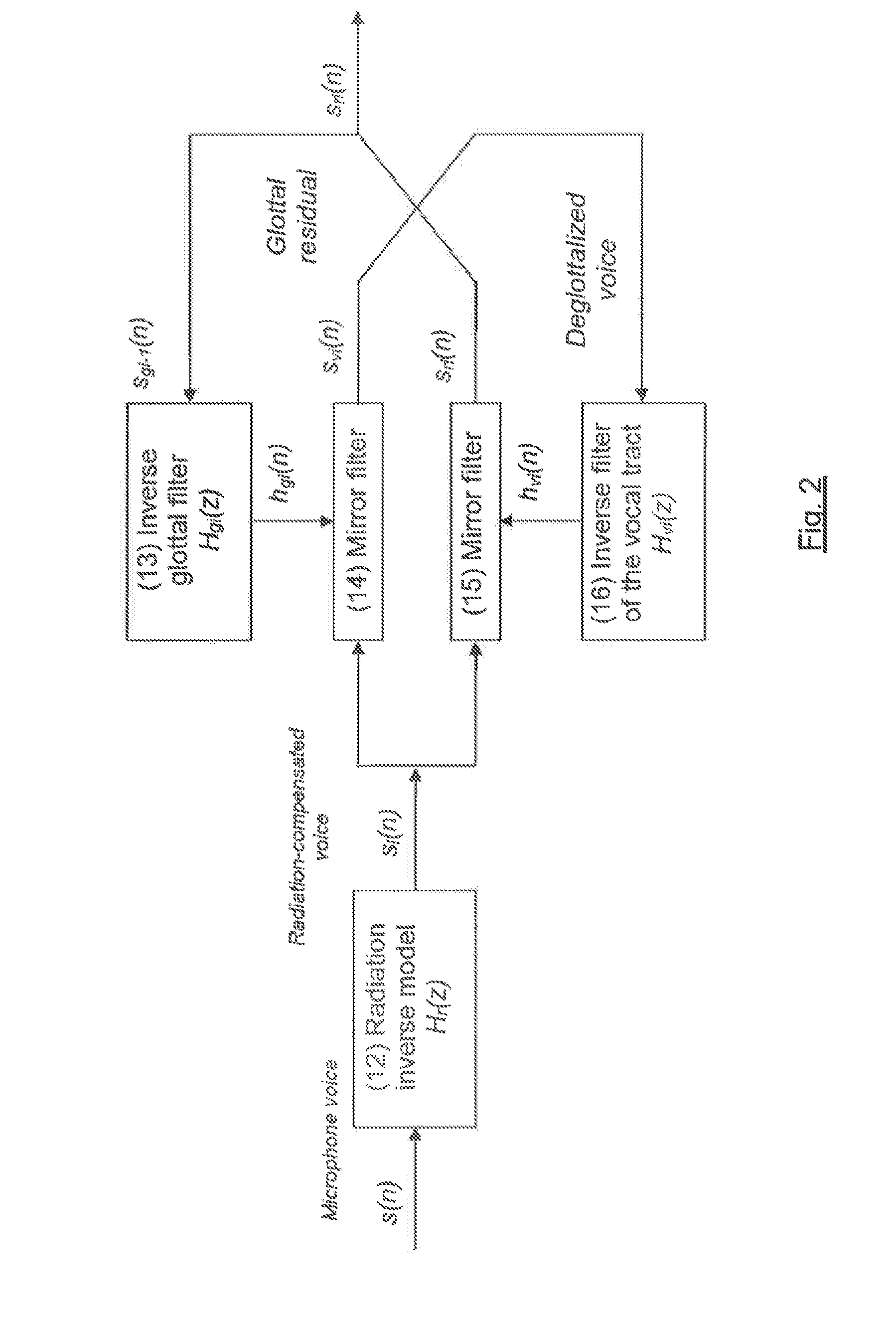
Method and system for estimating physiological parameters of phonation
The invention consists of a method and computing system for recording and analyzing the voice which allows a series of parameters of phonation to be calculated. These transmit relevant information regarding effects caused by organic disorders (which affect the physiology of the larynx) or neurological disorders (which affect the cerebral centers of speech). The classification methods are also considered an essential part of the invention which allow estimations of the existing dysfunction to be obtained and for the allocation of personality. The usefulness of the invention lies in the possibility of applying the dysfunction estimation in primary care service centers for patient screening to specialist care centers, simplifying examination protocols, saving costs and reducing waiting lists. This methodology can also be used for detecting the personality of a speaker by their voice, allowing access to installations or services.
Owner:UNIV MADRID POLITECNICA
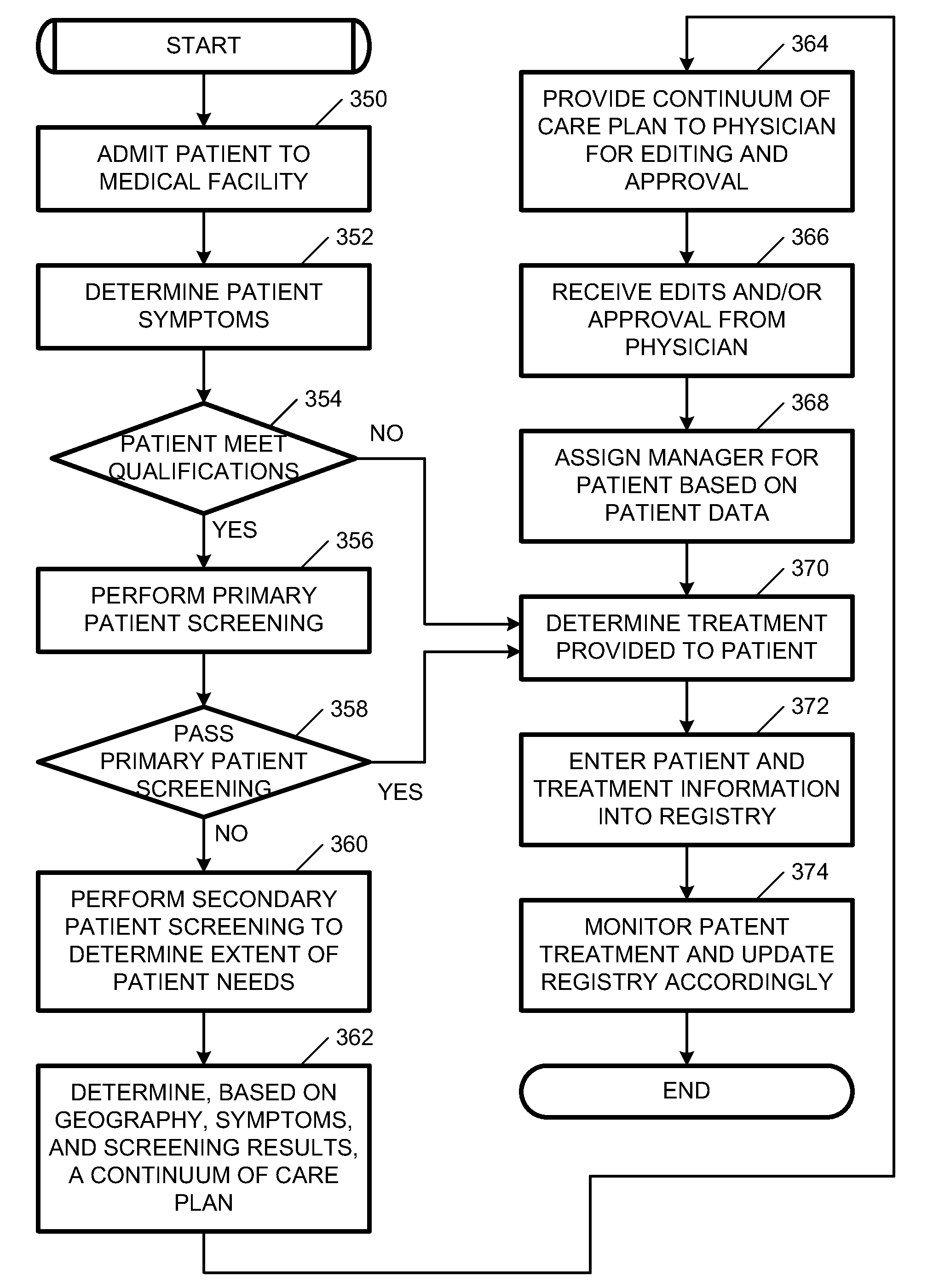
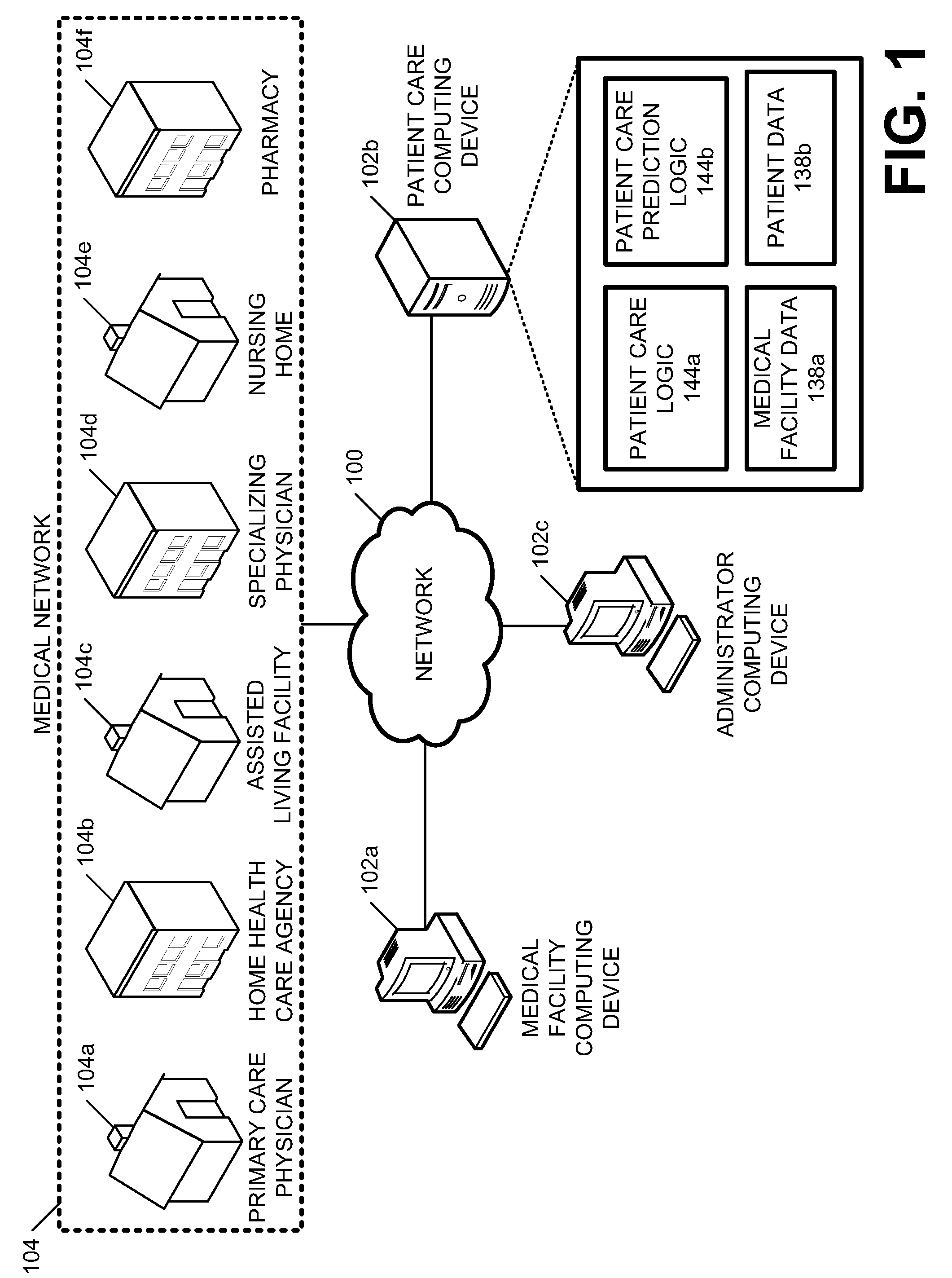
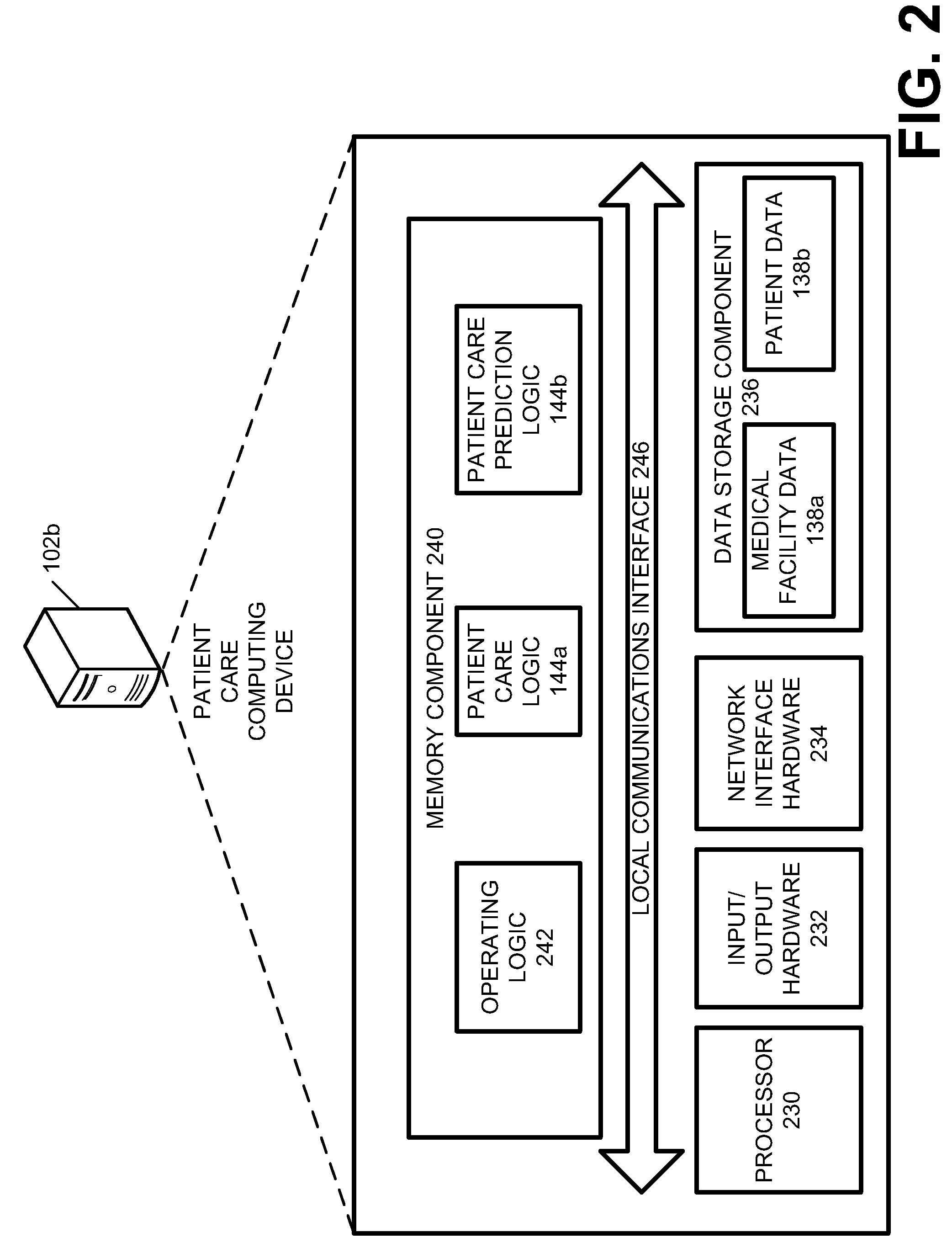
Systems and methods for providing a continuum of care
Disclosed herein are embodiments for providing a continuum of care. At least one embodiment includes receiving patient information for a patient, the patient information including medical information of the patient suffering from a specific medical condition and determining whether the patient qualifies for the continuum of care. Similarly, some embodiments include, in response to determining that the patient qualifies for the continuum of care, facilitating a primary patient screening to determine whether the patient has a tangential medical condition. Still some embodiments include creating a continuum of care plan for the patient that utilizes the patient information and results of the primary screening to predict a future medical care need of the patient.
Owner:ROMANO DAVID C MD +2
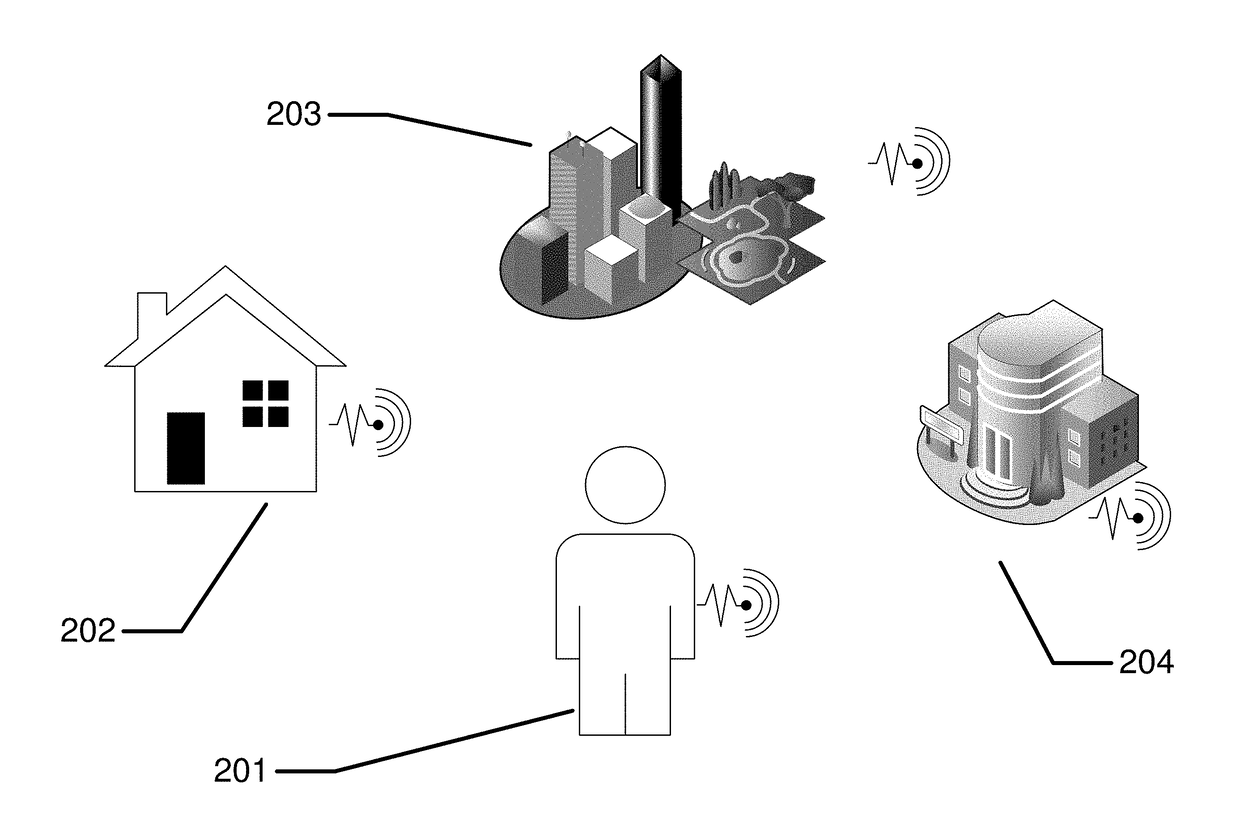
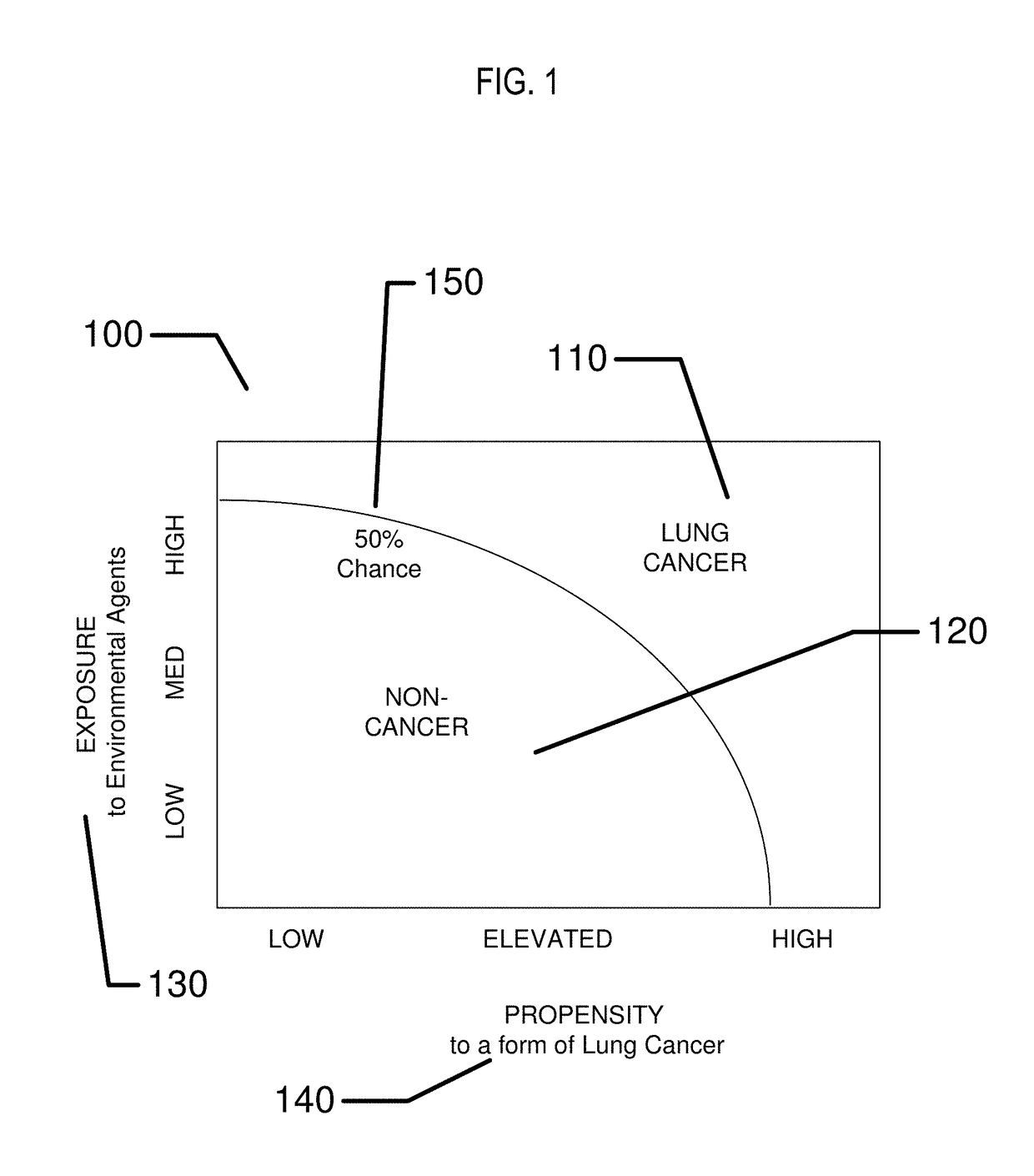
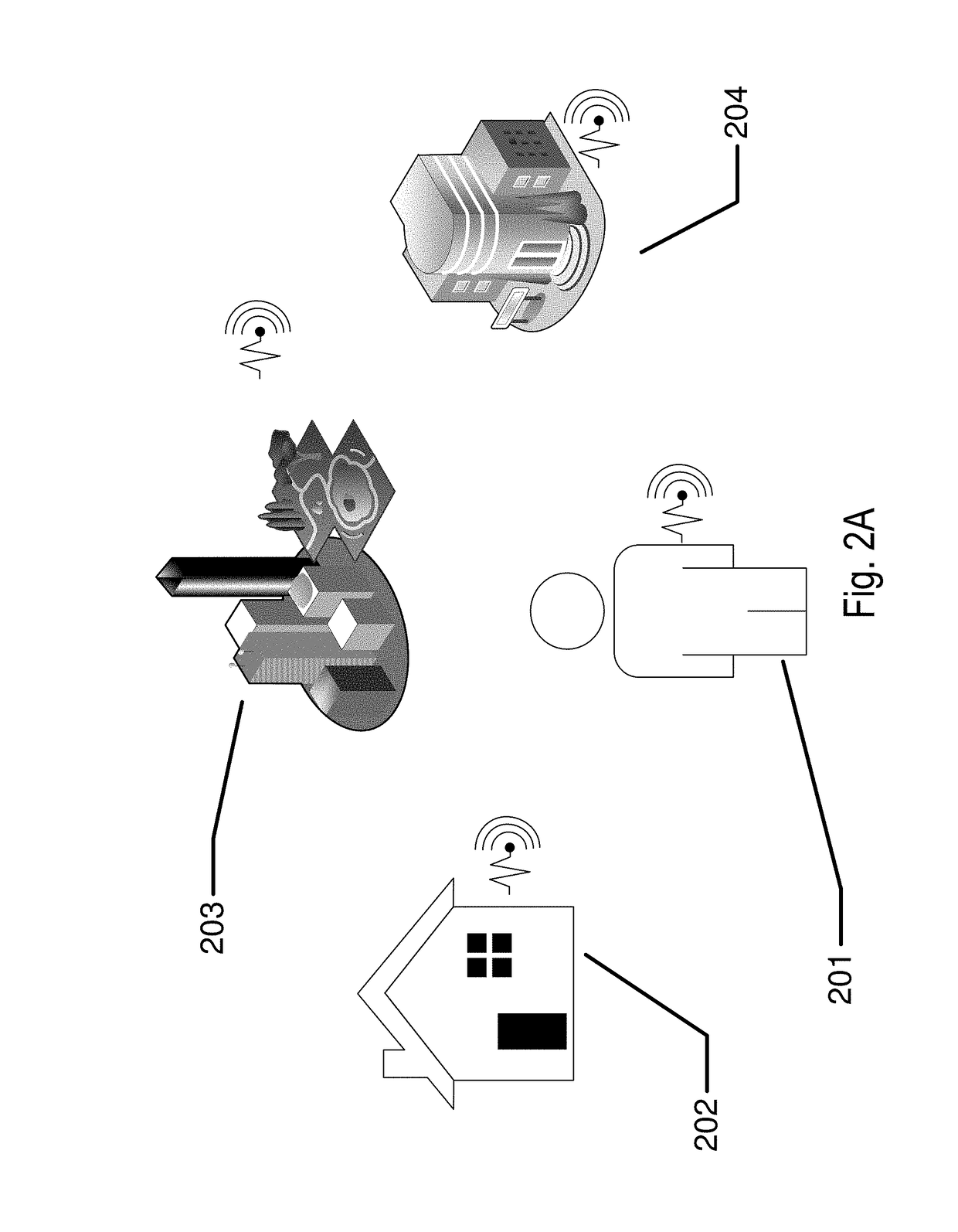
Biomedical sensing methods and apparatus for the detection and prevention of lung cancer states
InactiveUS20180144092A1Useful in detectionReduce riskRespiratory organ evaluationCheminformatics programming languagesEnvironmental healthEnvironmental agent
The present invention relates to methods and apparatus to monitor and potentially prevent occurrences of lung cancer in patients. The invention includes apparatus and methods to determine a patient's pre-disposition or propensity to acquire lung cancer. Also included are apparatus and methods to monitor for patient biomarkers and for environmental agents, to warn and to take action to reduce exposure to environmental agents and to increase patient screening for the occurrence of lung cancer.
Owner:JOHNSON & JOHNSON VISION CARE INC
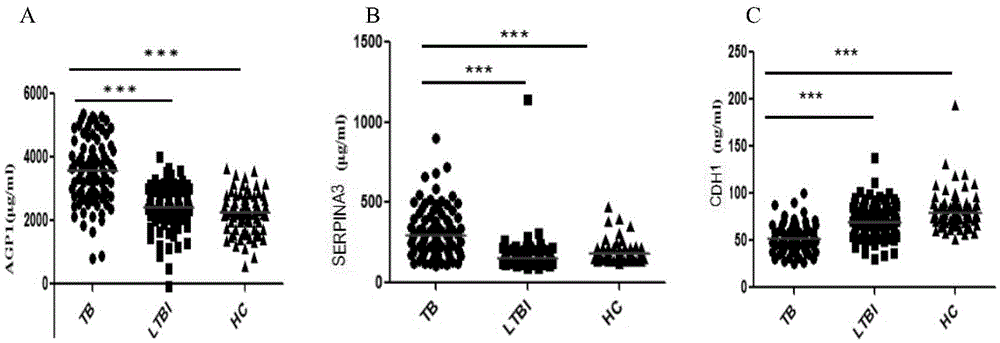
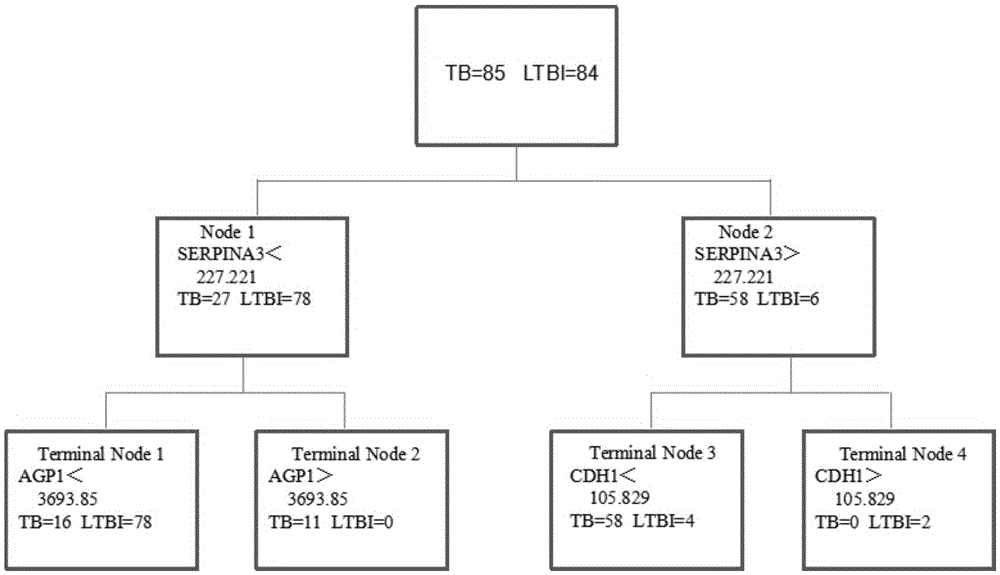
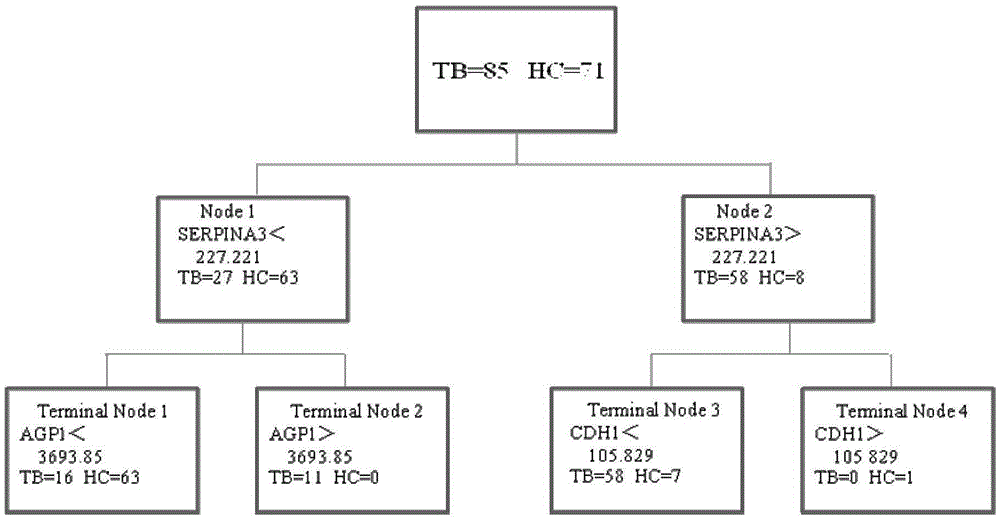
Application of AGP1, SERPINA3 and CDH1 content detection system in screening active tuberculosis patients
The invention discloses application of an AGP1, SERPINA3 and CDH1 content detection system in screening active tuberculosis patients. The inventor of the application discovers that three proteins with specific expression, namely AGP1, SERPINA3 and CDH1, in the active tuberculosis patients and establishes an active tuberculosis patient screening model with sensitivity of 81.18%, specificity of 96.15% and accuracy of 88.17% by use of content of three proteins in plasma. By use of the content of three proteins in plasma and the active tuberculosis patient screening model, the active tuberculosis patients can be screened, and active tuberculosis patient screening products can be prepared by use of substances for detecting the content of three proteins in plasma.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV +1
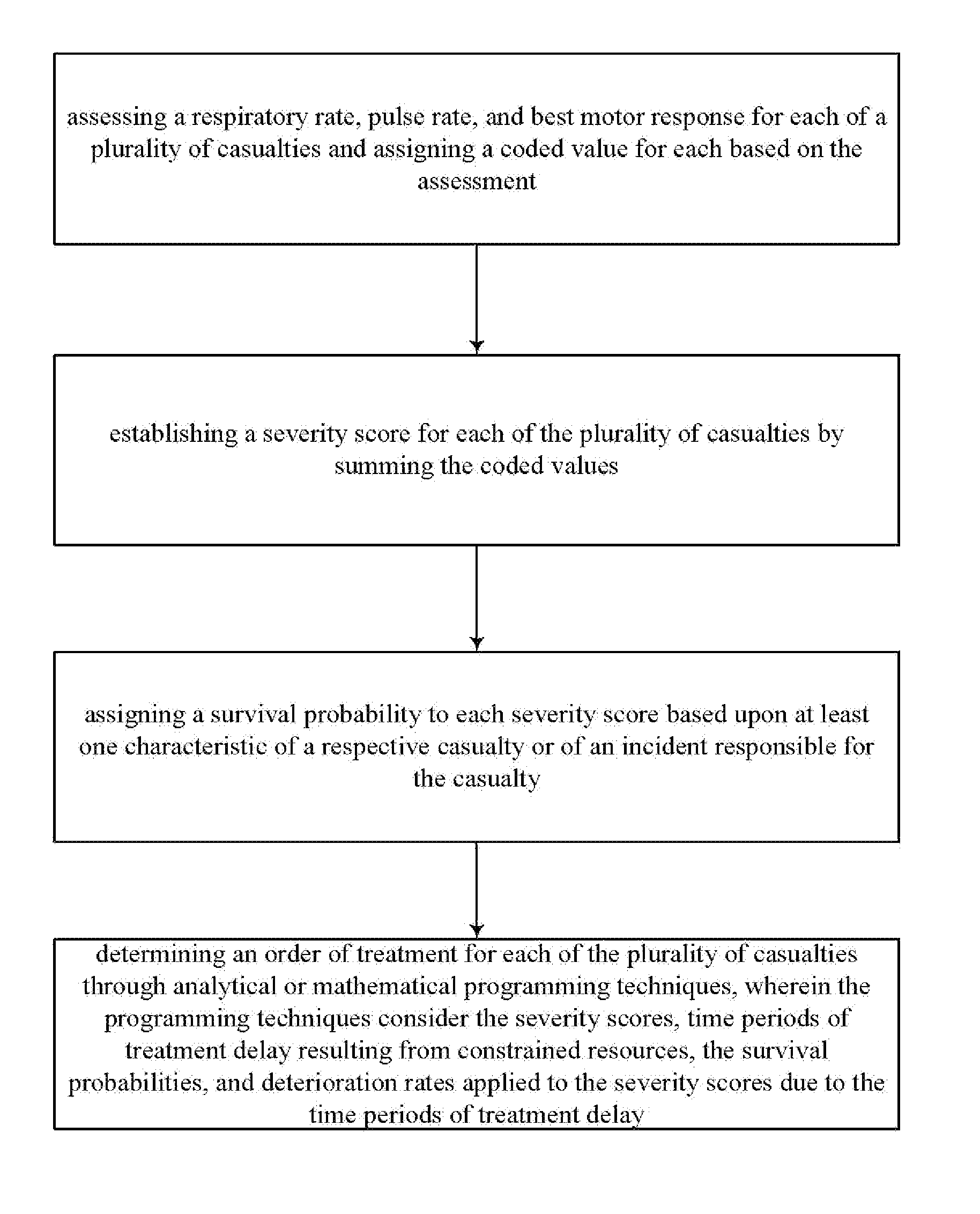
Transportation mode determination in non-mass casualty triage
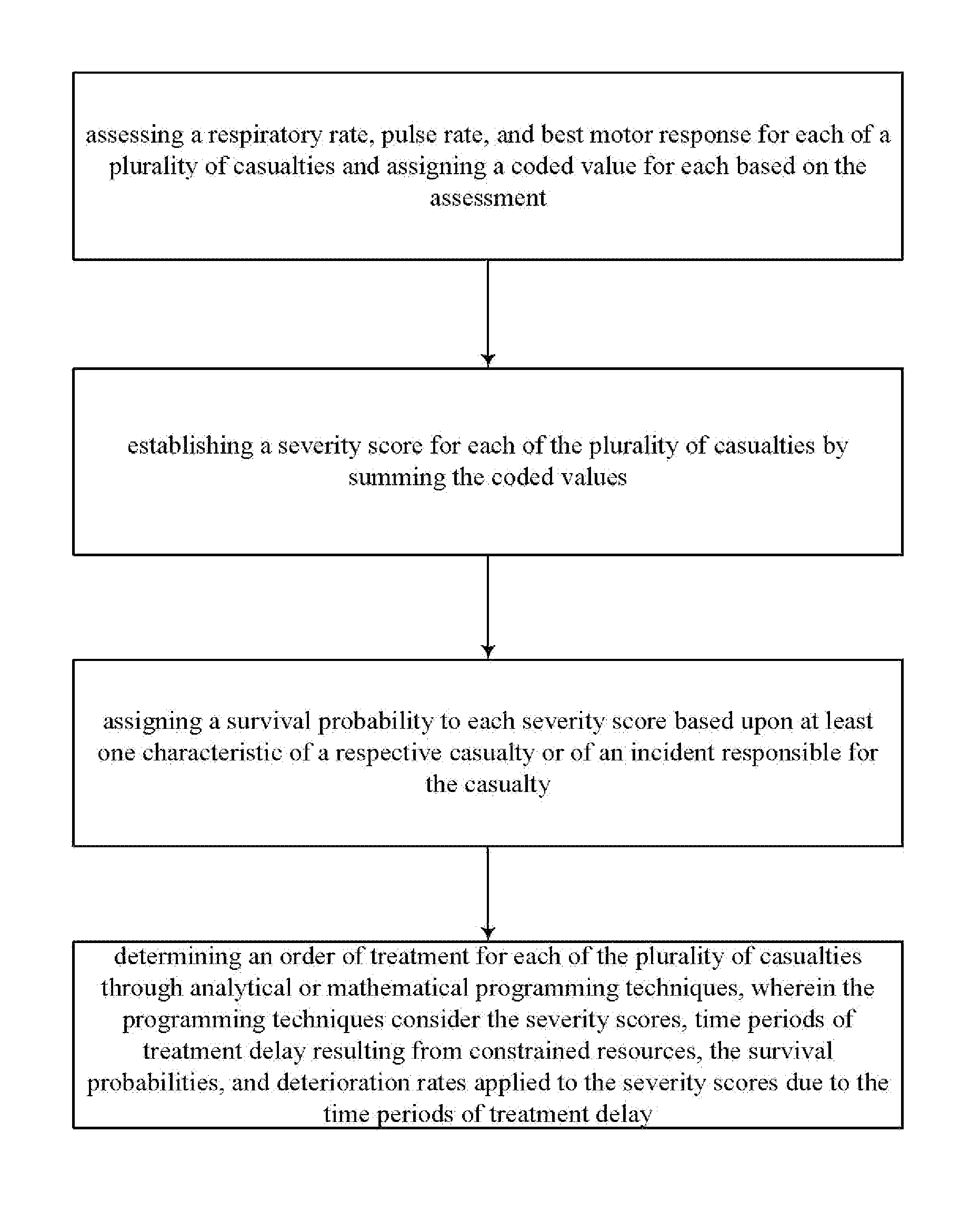
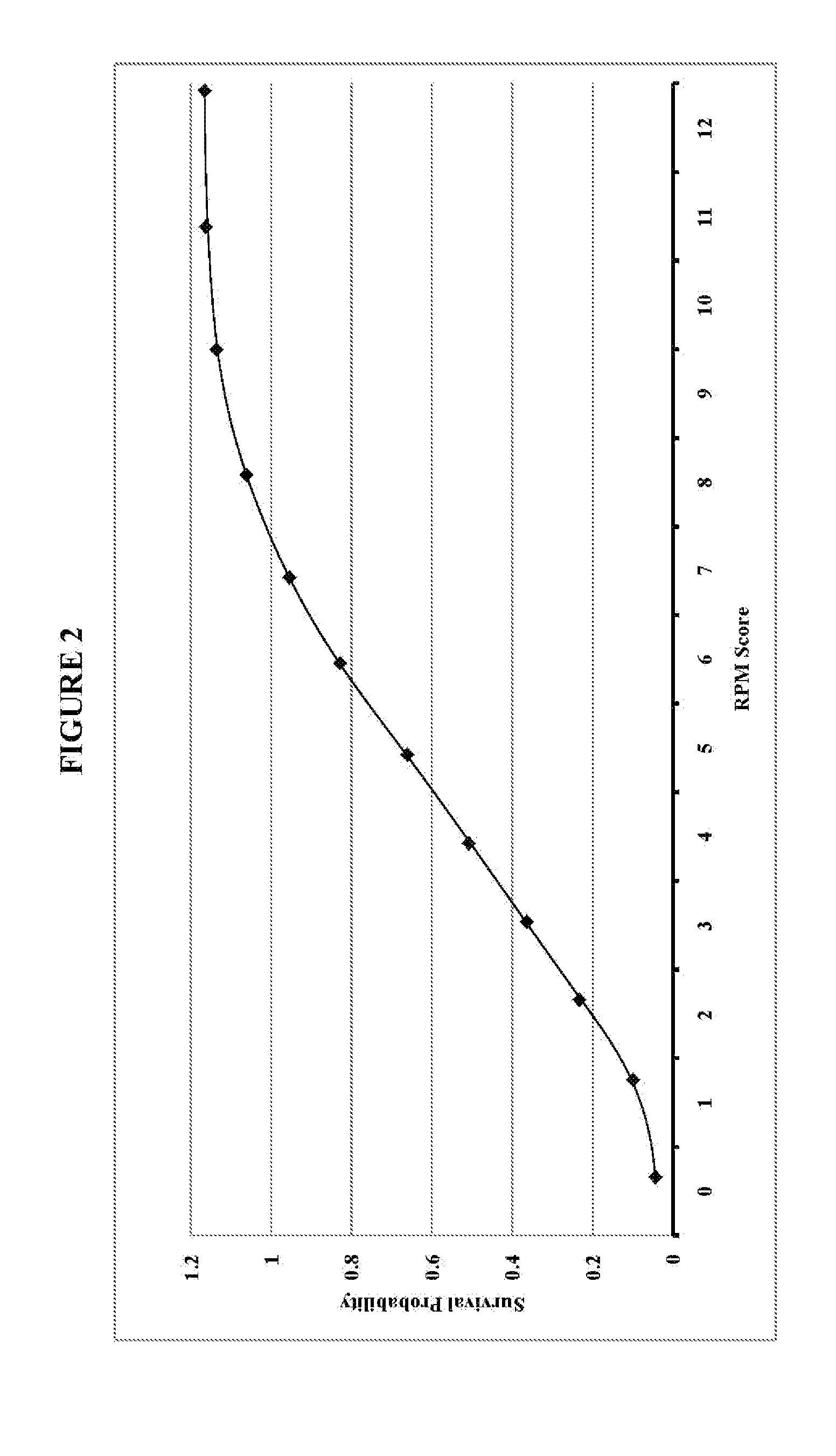
InactiveUS20110313783A1Maximizing numberMaximize survivabilityHealth-index calculationEpidemiological alert systemsTriageTrauma center
Transportation mode determination in non-mass casualty triage is provided and involves receiving, from incident personnel at a casualty scene, patient screening information for a victim or victims at the casualty scene. Location screening information for the victim or victims is also established and received. Victim eligibility for Medevac transport is determined by logically factoring the patient screening information and the location screening information. The determination can be performed by computer implemented decision logic. In addition to determining eligibility of the victim or victims for Medevac transport, transportation mode determination (e.g., what type of transportation) can be established for those determined eligible, determination of whether to transport to a trauma center or care facility, determination / selection of a specific trauma center or care facility for treatment, and / or a determination of an order of treatment, or transport for treatment, for each of the victims.
Owner:THINKSHARP
Vision screener
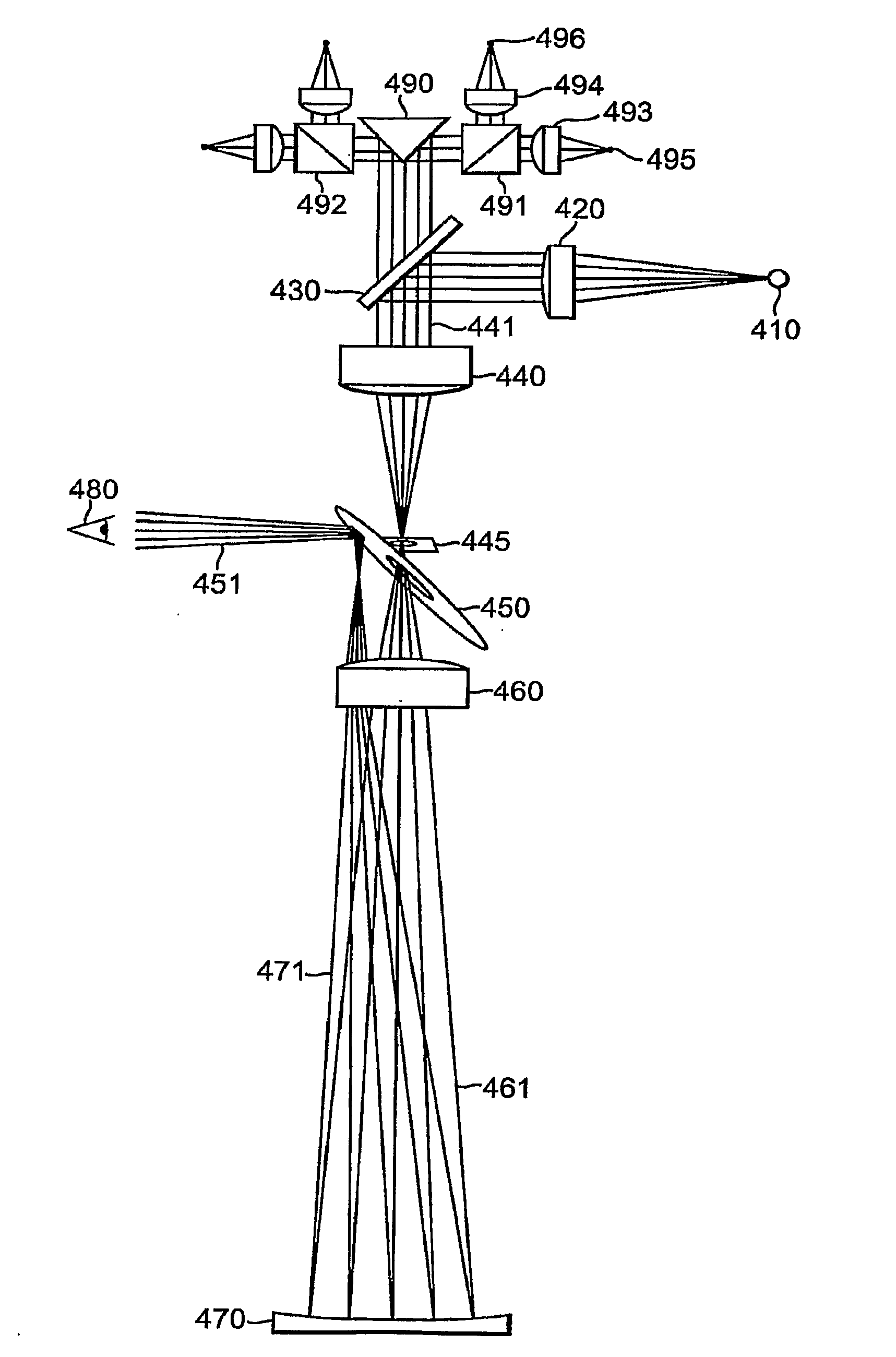
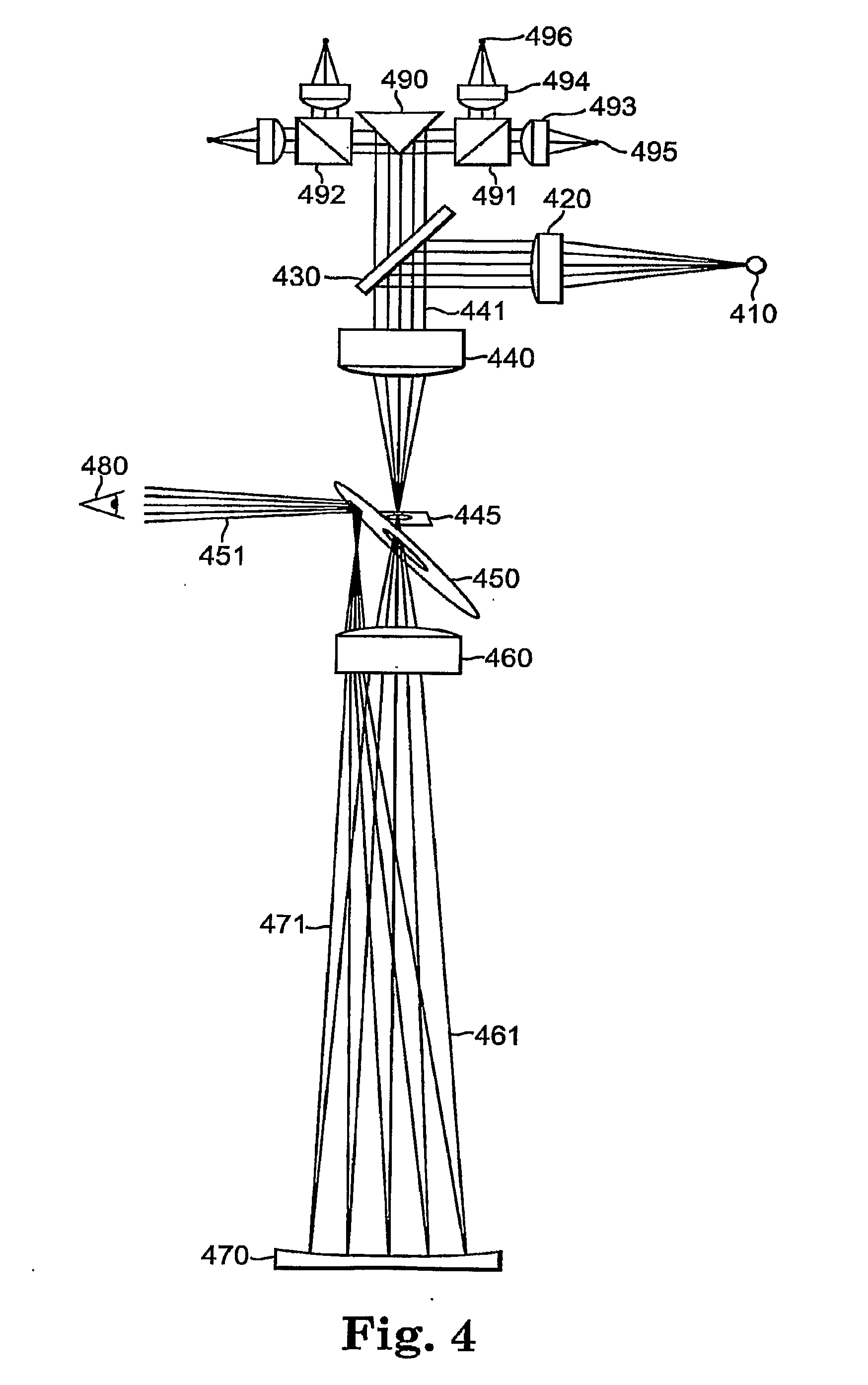
Generally, the present invention relates to medical devices and a method of vision screening, and more particularly to a pediatric vision screening system and method thereof that identifies a risk factor for amblyopia or diagnoses amblyopia by measurement of microstrabismus. An embodiment of the invention is directed to a method of patient screening for risk factors for amblyopia which includes the steps of illuminating the eye with polarized light, scanning the polarized light about the eye, capturing the retro-reflected light emanating back from the eye, analyzing the retro-reflected light to determine ocular misalignment; and calculating a metric to determine if the patient passes or fails the screening test thereby providing an indication that the patient may have a risk of amblyopia based on either strabismus or anisometropia. The method is effective at detecting amblyopia related to focusing problems without the measuring the focus of the eye directly.
Owner:CHILDRENS MEDICAL CENT CORP
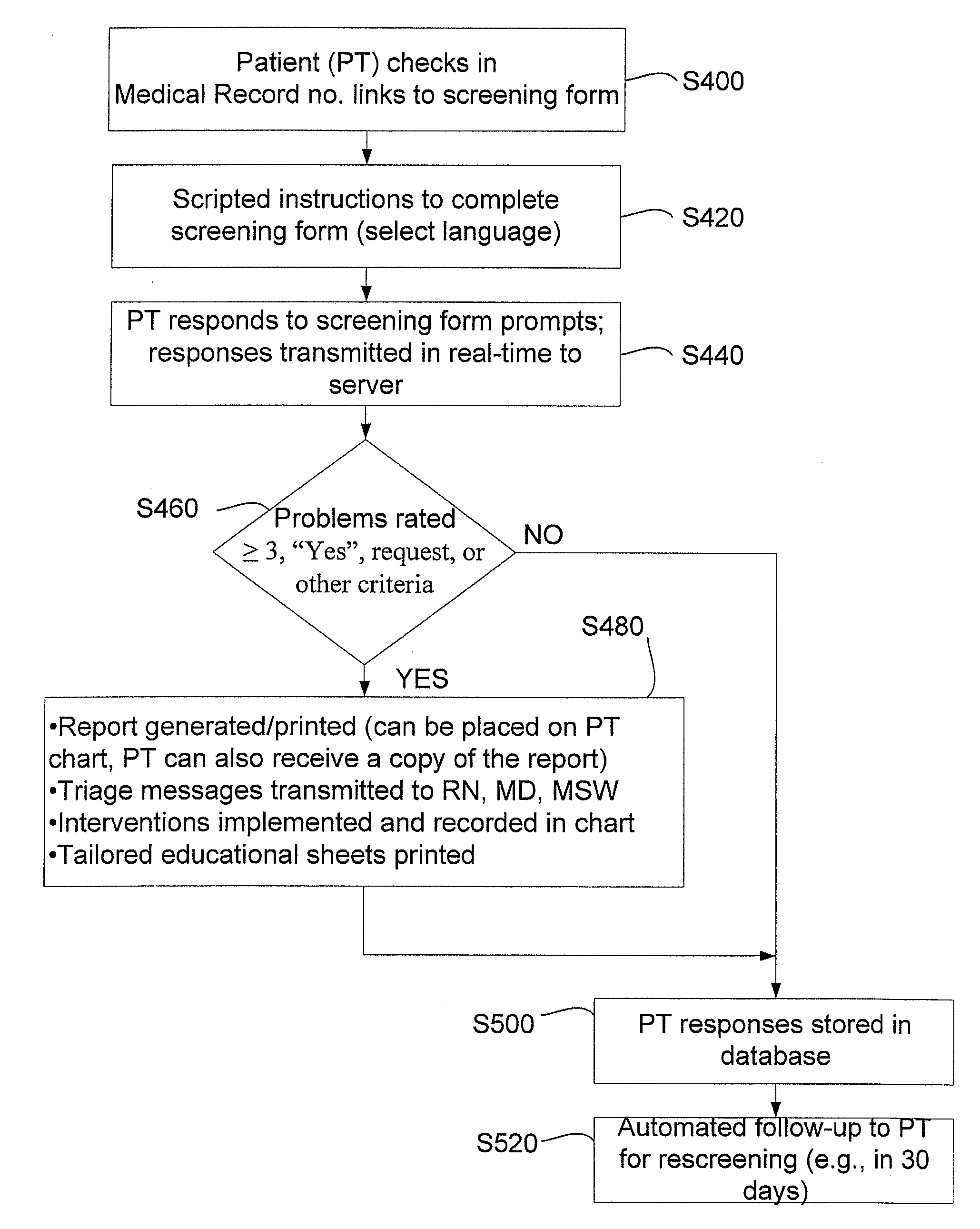
Method, apparatus and system for automated patient screening and triage
InactiveUS20110295620A1Data processing applicationsComputer-assisted medical data acquisitionTriageComputer printing
A computer-based screening instrument system and method comprising a patient interactive device coupled with a data network and an administrative server, the administrative server operatively connected to a database and a printer, wherein the administrative server is configured to serve as a screening instrument to the patient interactive device and to receive responsive information from the patient interactive device; and a triage module, wherein the screening instrument comprises a plurality of issues, an issue having a patient prompt and one or more patient selectable responses, wherein the patient interactive device, for each issue in the screening instrument, displays the related patient prompt, receive an indication of a patient selection of a related patient selectable response and transmit information representing the indication to the administrative server; wherein the administrative server receives the information and stores a patient response record comprising the information received; wherein the triage module compares a particular patient's response record with a set of triage rules and performs at least one of the following: (1) generate and transmit a triage message indicating a patient exigency to a communication device of a member of the patient's professional health care team, and (2) generate and transmit a message indicating the patient's request to discuss an issue with a member of the patient's professional health care team to the communication device.
Owner:CITY OF HOPE
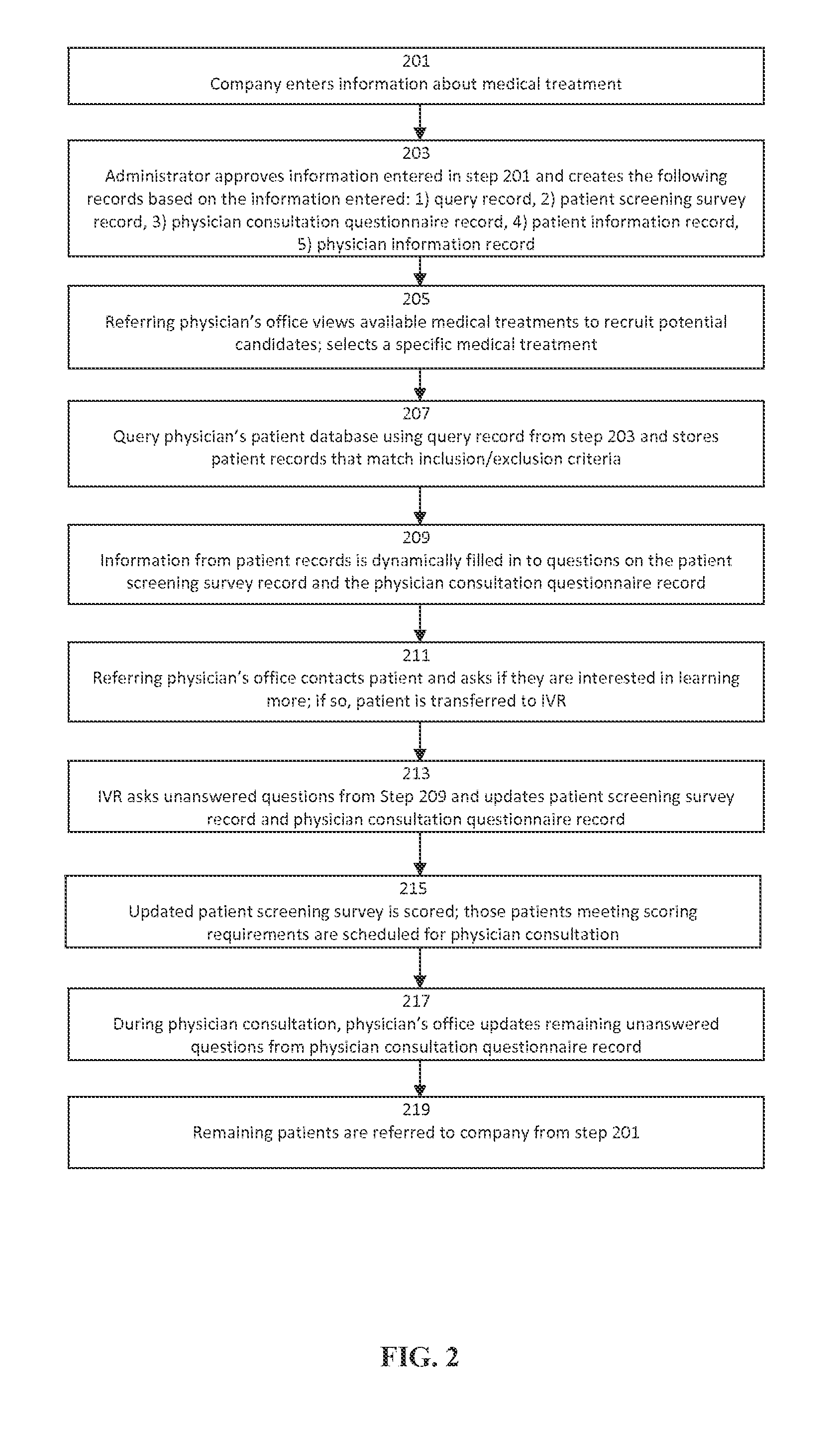
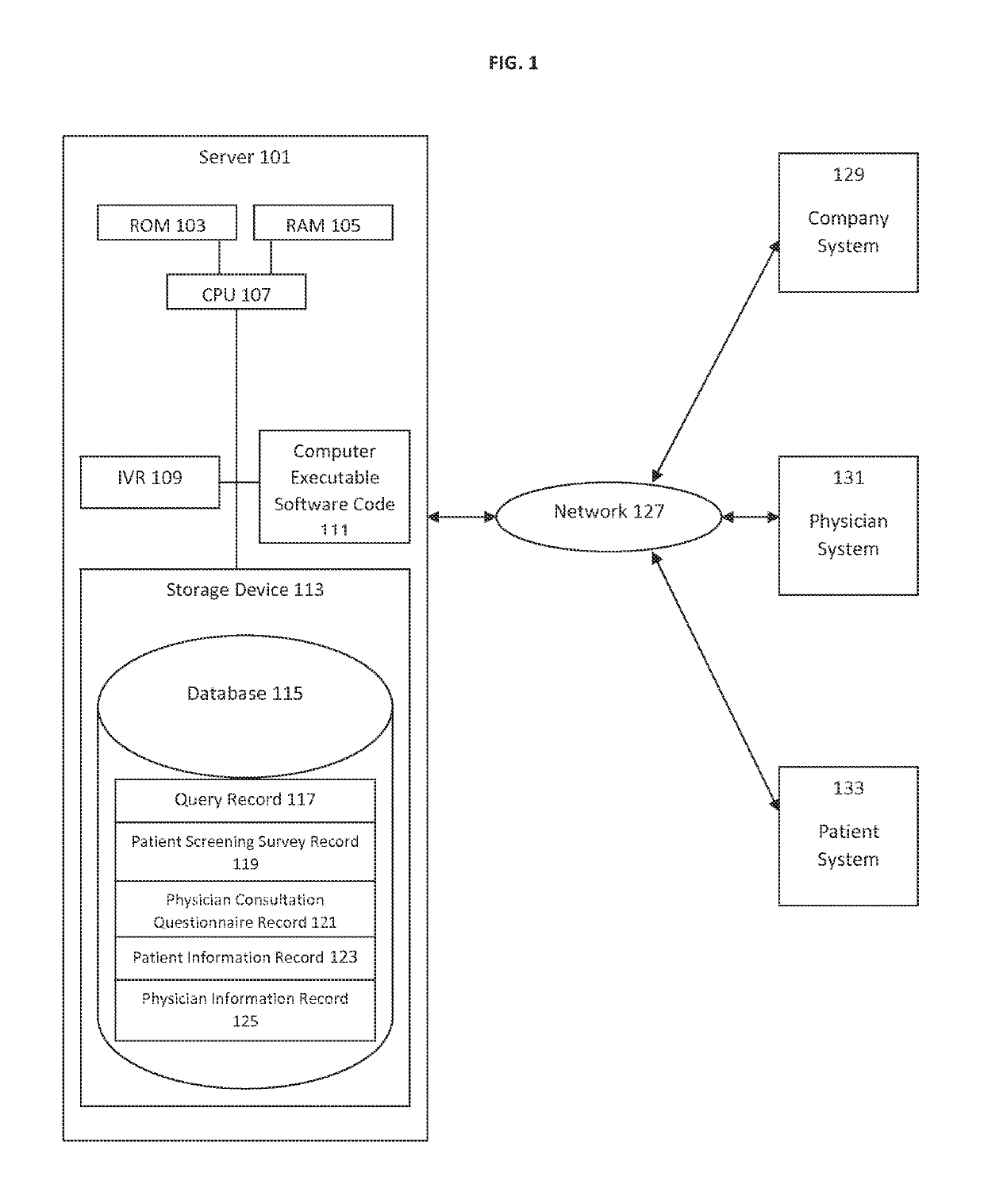
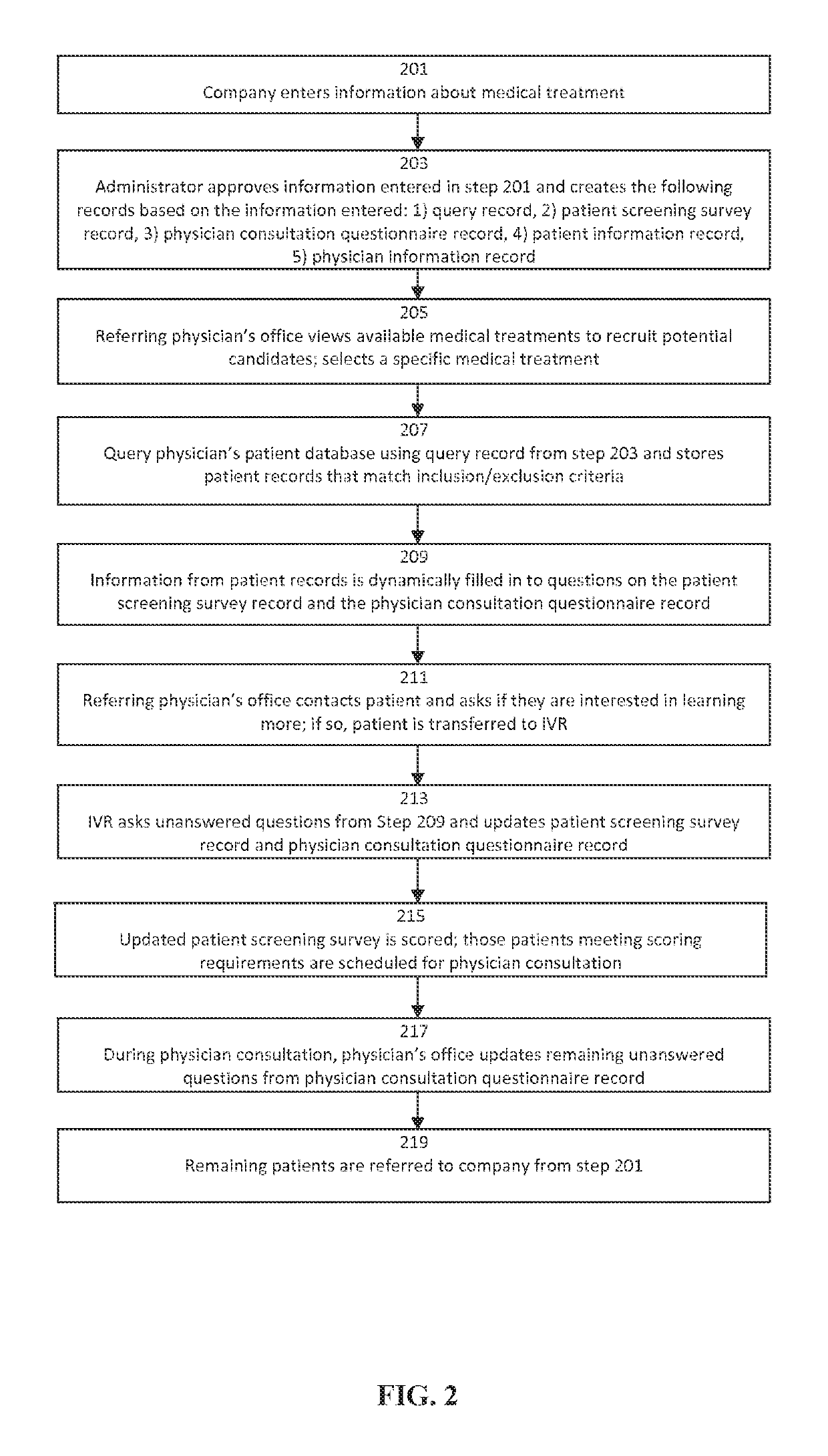
Predictive Patient to Medical Treatment Matching System and Method
InactiveUS20150213234A1Effective and efficient identificationEfficient identificationData processing applicationsComputer-assisted medical data acquisitionPredictive systemsPatient database
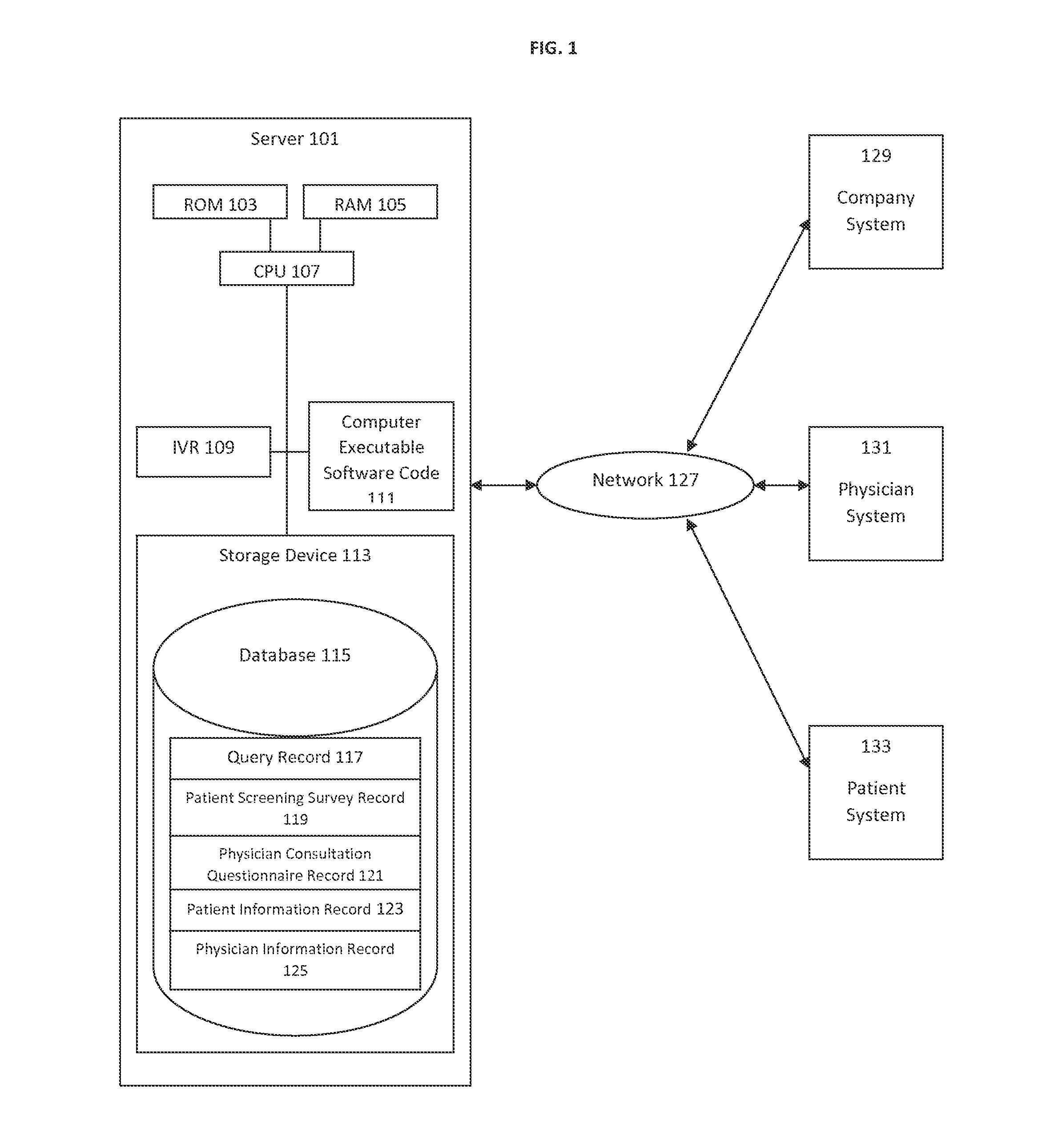
A predictive system and method for using a physician's existing patient database to more effectively and efficiently identify potential candidates for medical treatments or clinical research trials. The system and method includes an automated three-tiered qualification process using information about a specific medical treatment received from a company looking for potential candidates for the medical treatment wherein potential candidates are initially prequalified by comparing the information about the medical treatment to the information contained in a physician's patient database. The potential candidates that are identified by the first tier of the qualification process then answer additional questions from a patient screening survey. Finally, additional questions from a physician consultation questionnaire are completed by the referring physician's office. If a potential candidate passes all three tiers of qualification, the potential candidate is then passed to the company looking for potential candidates for the medical treatment.
Owner:ELLIGO HEALTH RES INC
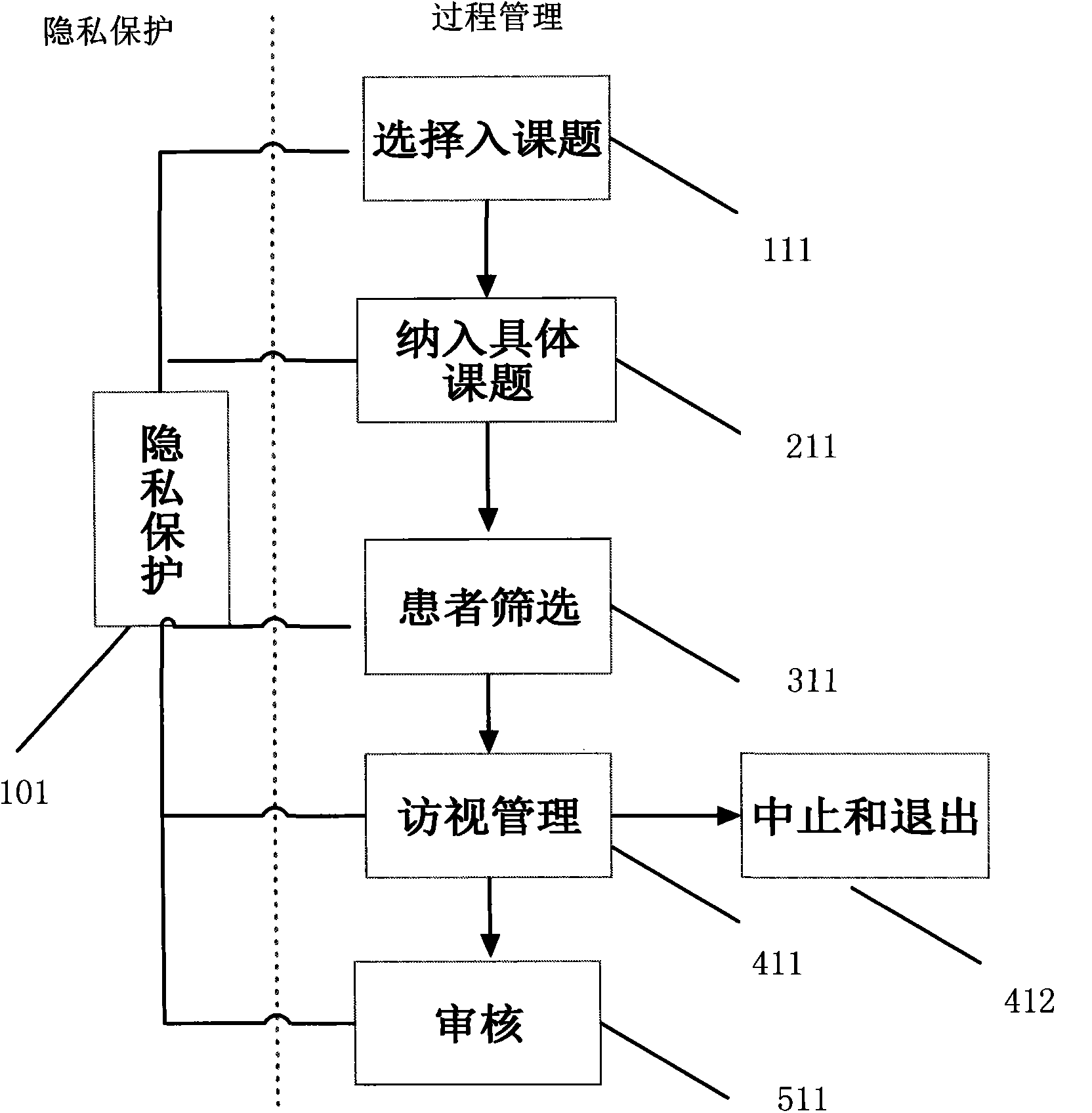
Patient administration system for real world clinical study
InactiveCN103761698AImplement system managementImprove complianceData processing applicationsPatient-specific dataResearch qualityPatient compliance
The invention discloses a patient administration system for a real world clinical study. The system is applied to patient administration in the real world clinical study. According to the system, systematic administration of patients is achieved through determination of patient participation in a project, patient screening, visiting administration, patient drop-out administration, patient privacy protection and the like, and the purposes of improving research quality and patient compliance are realized.
Owner:CHINESE ACAD OF PREVENTIVE MEDICINE
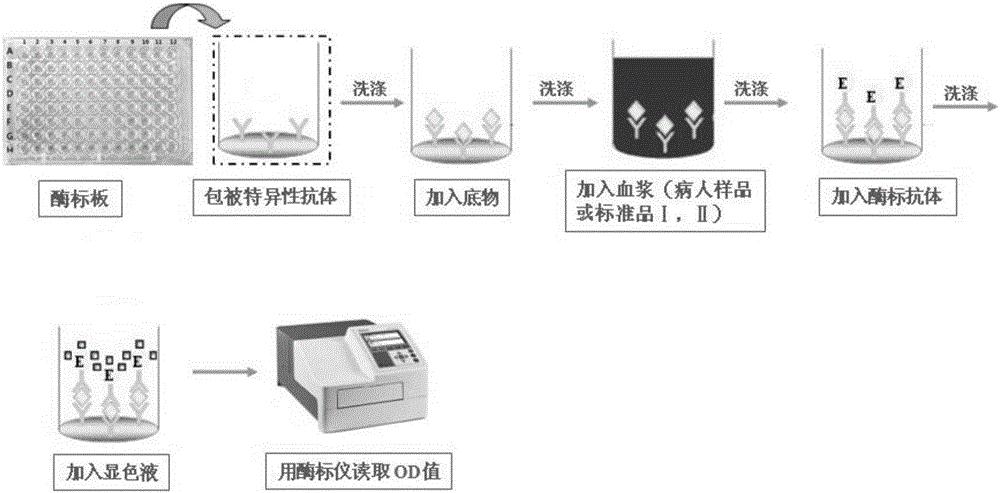
Substrate, method and kit for in-vitro quantitative determination of activity of vWF-CP enzyme
The invention relates to the biological field, and especially relates to a substrate, a method and a kit for in-vitro quantitative determination of the activity of vWF-CP enzyme. The substrate comprises a vWF functional area amino acid sequence. The end N and the end C of the vWF functional area amino acid sequence are respectively combined with a HIS label amino acid sequence and a FLAG label amino acid sequence. The method comprises the following steps: the holes of an ELISA plate are coated with an antibody in advance; and then, the substrate, a sample to be tested, an ELISA antibody, and chromogenic agent are added, and the absorbance of liquid in the holes of the ELISA plate is measured using an ELISA reader. The kit comprises an ELISA plate, a substrate, diluent, washing liquid, buffer solution, an enzyme-labeled antibody, chromogenic solution and stop solution, or comprises an ELISA plate, an antibody, a substrate, diluent, washing liquid, buffer solution, an enzyme-labeled antibody, chromogenic solution and stop solution. The substrate, the method and the kit for in-vitro quantitative determination of the activity of vWF-CP enzyme have the advantages of high sensitivity, high accuracy, simple operation and low price, and are suitable for broad patient screening.
Owner:吴江华药生物技术有限公司
Method of risk management for patients undergoing Natalizumab treatment
Progressive multifocal leukoencephalopathy (PML) has been identified in patients taking natalizumab (NMAB) for the treatment of multiple sclerosis (MS). This patent application provides a novel method of patient screening and monitoring intended to decrease the risk of PML and other opportunistic central nervous system (CNS) diseases in patients undergoing MS therapy with NMAB, and proposes a novel method of screening and monitoring intended to decrease the risk of opportunistic disease processes of the CNS during the treatment of other medical disorders with NMAB.
Owner:SEEDLINGS LIFE SCI VENTURES
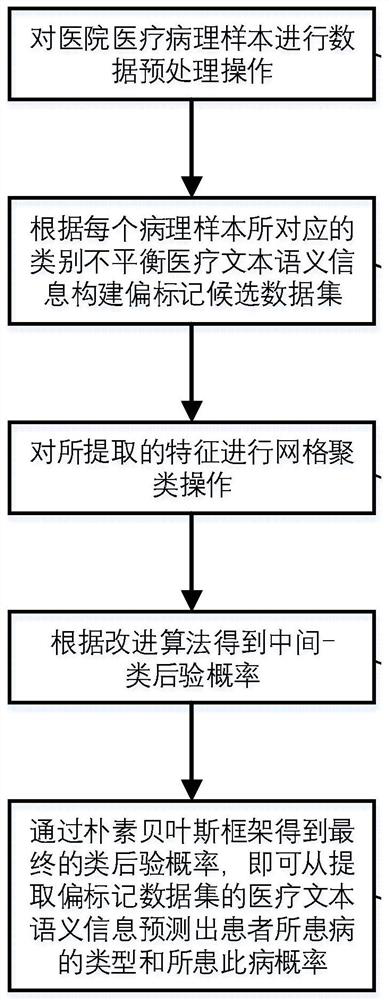
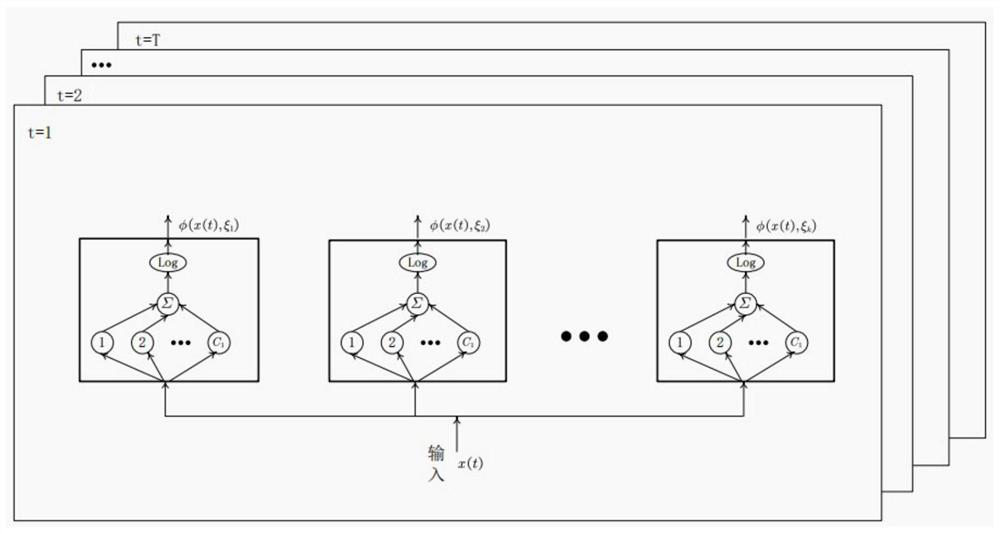
Patient screening and marking method based on partial multi-mark learning
PendingCN114093445AMitigate learning performance impactImprove classification performanceMathematical modelsMedical data miningDiseaseMedical treatment
The invention belongs to the field of partial multi-mark learning and data mining, and particularly relates to a patient screening and marking method based on partial multi-mark learning. The method comprises the following steps: acquiring pathological sample data of a patient, inputting the pathological sample data into a trained medical text semantic information big data prediction model based on partial multi-mark learning, predicting a disease type and a disease probability of the patient, and marking the patient according to the disease type and the disease probability of the patient. According to the method, the problem of classification class imbalance is further processed, a more accurate marking result can be predicted, a patient can perform health management according to the marking result, a doctor can perform next diagnosis on the patient according to the result, and the method has good social benefits and economic benefits.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
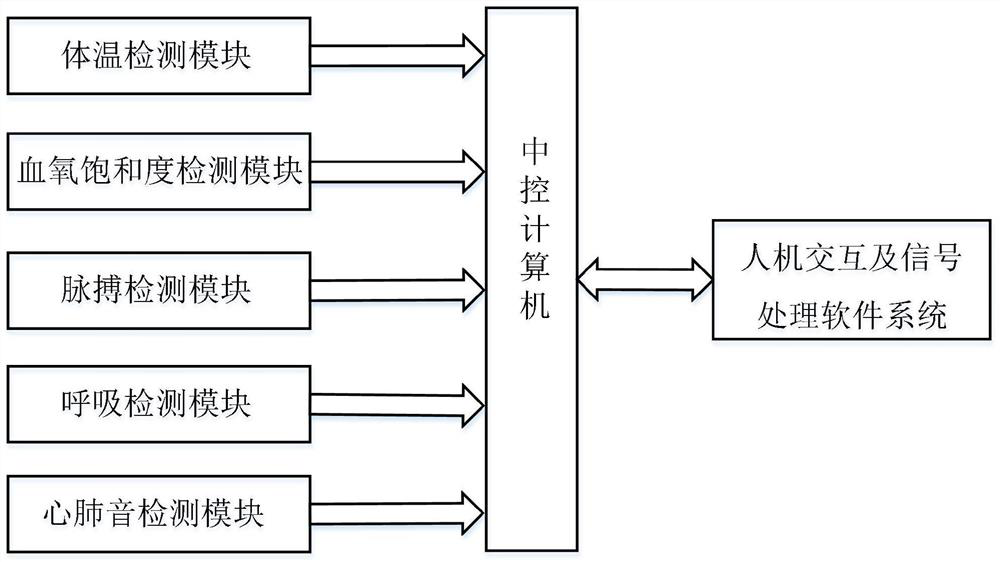
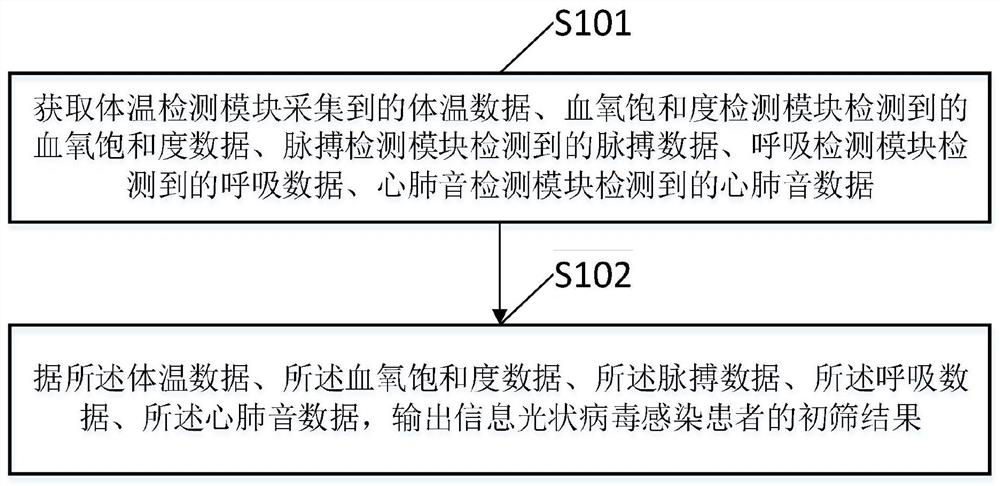
Novel coronavirus infected patient rapid preliminary screening detector and detection method
PendingCN111603144AUnderstand Physiological ConditionsRealize automatic diagnosisAuscultation instrumentsSensorsRespiratory rateCoronavirus
The invention relates to a novel coronavirus infected patient rapid preliminary screening detector and a detection method, and belongs to the field of medical detection. The system is on the same instrument platform. Physiological and pathological signals such as individual body temperature, oxyhemoglobin saturation, pulse rate, respiratory rate and cardiopulmonary sound are synchronously detected, characteristic analysis is carried out on each detected signal, and a novel coronavirus infection automatic diagnosis model is constructed, so that rapid and accurate novel coronavirus infected patient screening is realized, the missed diagnosis risk caused by the fact that part of novel coronavirus infected patients do not have obvious symptoms and only depend on body temperature for primary screening is overcome, and the novel coronavirus epidemic prevention and control capacity is improved. The system has the characteristics of high intelligence degree, high integration degree, good portability and the like, screening and treatment effect evaluation of novel coronavirus pneumonia infected patients in different scenes can be effectively improved, and the epidemic prevention and treatment requirements are better met.
Owner:中彦医疗科技有限责任公司
Biomarker related to specific immunotherapy of dust mite related asthma of children and application
ActiveCN111443206ACause some damagesAvoid economic lossDisease diagnosisBiological testingSpecific immunityCurative effect
The invention discloses a biomarker related to specific immunotherapy of dust mite related asthma of children and application. The biomarker is keratin 1, and the invention also discloses applicationof the biomarker to preparation of a reagent, a kit, test paper or a chip for auxiliary screening of patients of specific immunotherapy of the dust mite related asthma of the children. According to the biomarker and the application of the biomarker, the blank that a biomarker which can be conventionally used for immunotherapy patient screening and curative effect pre-judgment is still lacked at present is filled up; and the biomarker is closely related to the specific immunotherapy of the dust mite related asthma of the children, is beneficial to accurate selection of the children patients suitable for the specific immunotherapy, avoids economic losses and body injuries caused by immunotherapy of the children patients with poor curative effects, and has great application significance and economic value.
Owner:FOSHAN MATERNAL & CHILD HEALTH CARE HOSPITAL
Systems and Methods For Providing A Continuum Of Care
InactiveUS20120059672A1Medical automated diagnosisOffice automationPrimary screeningEmergency medicine
Disclosed herein are embodiments for providing a continuum of care. At least one embodiment includes receiving patient information for a patient, the patient information including medical information of the patient suffering from a specific medical condition and determining whether the patient qualifies for the continuum of care. Similarly, some embodiments include, in response to determining that the patient qualifies for the continuum of care, facilitating a primary patient screening to determine whether the patient has a tangential medical condition. Still some embodiments include creating a continuum of care plan for the patient that utilizes the patient information and results of the primary screening to predict a future medical care need of the patient.
Owner:ROMANO DAVID C MD +2
Transportation mode determination in non-mass casualty triage
InactiveUS8489419B2Maximizing numberMaximize survivabilityHealth-index calculationEpidemiological alert systemsTriageTrauma center
Transportation mode determination in non-mass casualty triage is provided and involves receiving, from incident personnel at a casualty scene, patient screening information for a victim or victims at the casualty scene. Location screening information for the victim or victims is also established and received. Victim eligibility for Medevac transport is determined by logically factoring the patient screening information and the location screening information. The determination can be performed by computer implemented decision logic. In addition to determining eligibility of the victim or victims for Medevac transport, transportation mode determination (e.g., what type of transportation) can be established for those determined eligible, determination of whether to transport to a trauma center or care facility, determination / selection of a specific trauma center or care facility for treatment, and / or a determination of an order of treatment, or transport for treatment, for each of the victims.
Owner:THINKSHARP
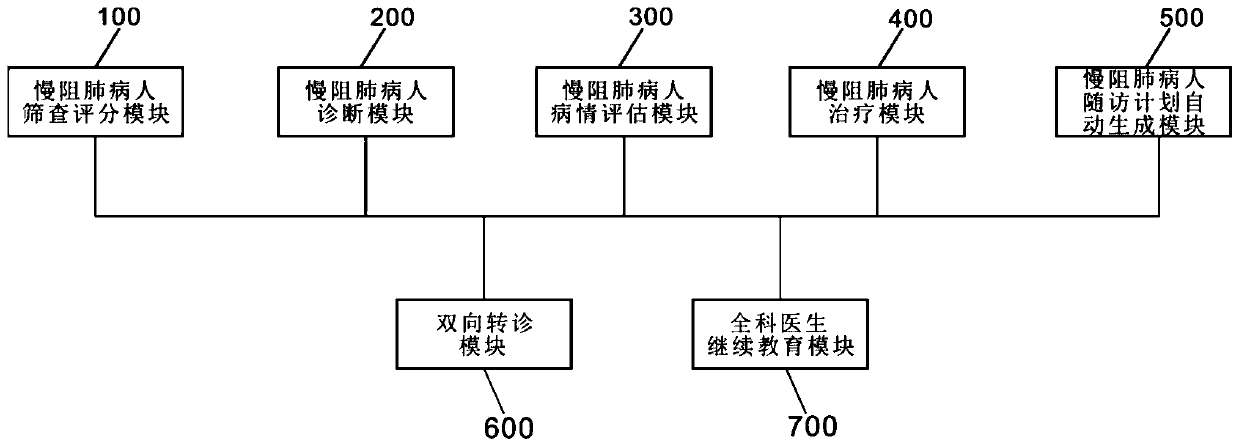
Hierarchical diagnosis-and-treatment information platform based on chronic obstructive pulmonary disease and diagnosis-and-treatment method
PendingCN110136821AAchieve early diagnosisRealize full monitoringMedical communicationMedical equipmentDiseaseObstructive Pulmonary Diseases
The invention provides a hierarchical diagnosis-and-treatment information platform based on a chronic obstructive pulmonary disease and a diagnosis-and-treatment method. The hierarchical diagnosis-and-treatment information platform comprises a chronic obstructive pulmonary disease patient screening scoring module, a chronic obstructive pulmonary disease patient blocking module, a chronic obstructive pulmonary disease patient evaluating module, a chronic obstructive pulmonary disease patient treatment module, a chronic obstructive pulmonary disease patient follow-up surveying plan automatic generating module, a bidirectional transfer diagnosis module and a general practitioner further education module. According to the hierarchical diagnosis-and-treatment information platform based on the chronic obstructive pulmonary disease and the diagnosis-and-treatment method, indexes related with diagnosis and evaluation can be effectively integrated and are displayed to the general practitioner;and then according to a standard disease diagnosis-and-treatment flow supplied by the hierarchical diagnosis-and-treatment information platform, the general practitioner can finish standard diagnosis-and-treatment of the chronic obstructive pulmonary disease patient, thereby uploading related data of the patient in a treatment period, realizing real-time dynamic understanding of the condition change of the patient, dynamically evaluating the treatment condition of the patient and supplying personalized standard treatment to the chronic obstructive pulmonary disease patient.
Owner:上海市松江区中心医院
Predictive patient to medical treatment matching system and method
InactiveUS10366780B2Effective and efficient identificationEfficient identificationPatient-specific dataElectronic clinical trialsPredictive systemsPatient database
A predictive system and method for using a physician's existing patient database to more effectively and efficiently identify potential candidates for medical treatments or clinical research trials. The system and method includes an automated three-tiered qualification process using information about a specific medical treatment received from a company looking for potential candidates for the medical treatment wherein potential candidates are initially prequalified by comparing the information about the medical treatment to the information contained in a physician's patient database. The potential candidates that are identified by the first tier of the qualification process then answer additional questions from a patient screening survey. Finally, additional questions from a physician consultation questionnaire are completed by the referring physician's office. If a potential candidate passes all three tiers of qualification, the potential candidate is then passed to the company looking for potential candidates for the medical treatment.
Owner:ELLIGO HEALTH RES INC
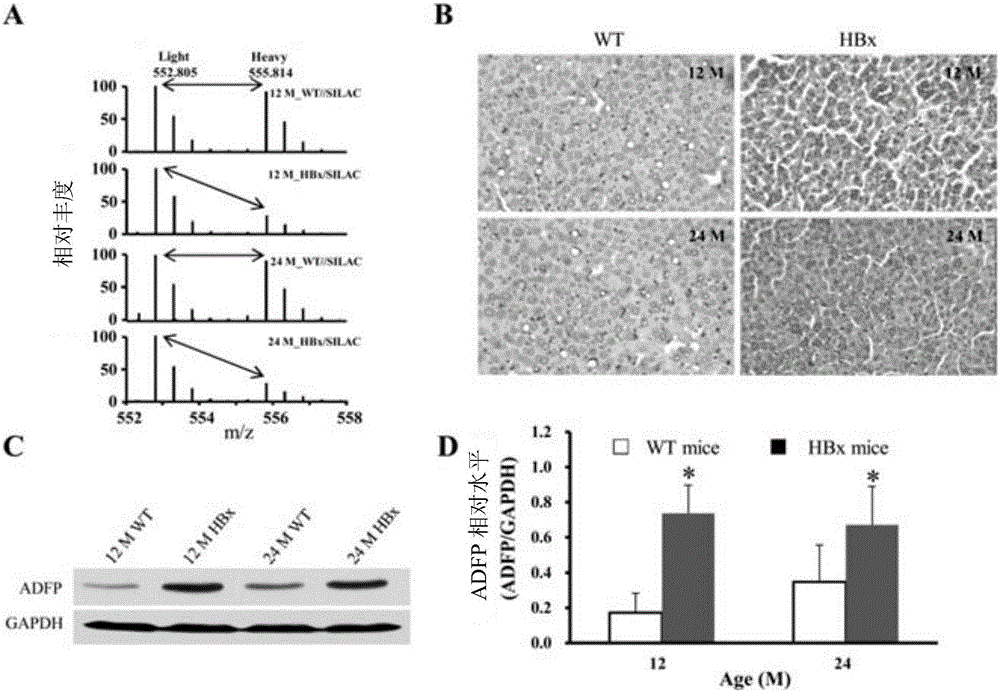
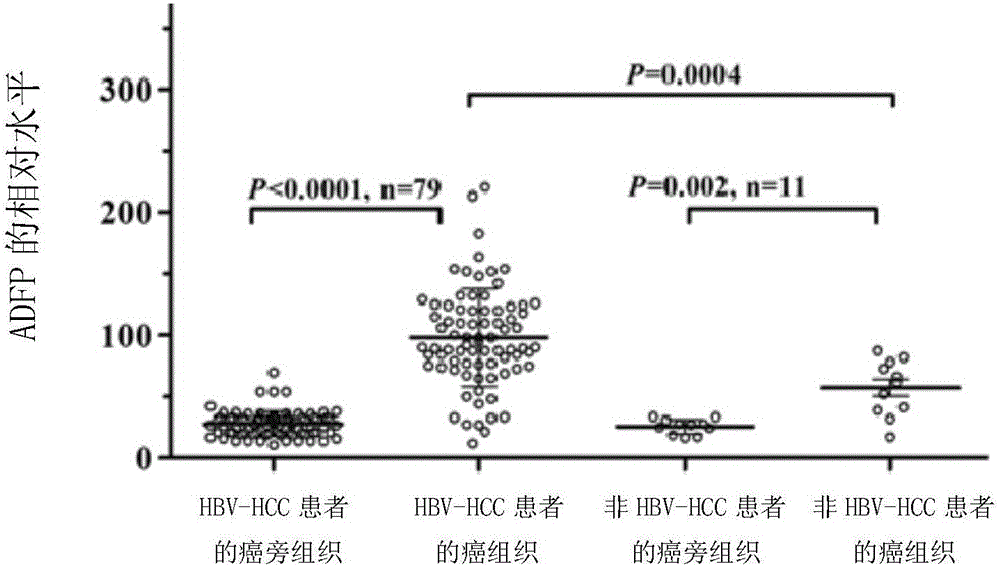
Novel use of adipogenic differentiation-associated proteins as screening markers for HBV-HCC patients
InactiveCN104931704BStrong specificityIncreased sensitivityBiological testingAdipose Differentiation-Related ProteinPatient screening
The invention discloses a novel application of adipose differentiation related protein as the HBV-HCC patient screening marker. The invention demands to protect the application of adipose differentiation related protein (ADFP) in preparation of kits for screening HBV-HCC patients. The invention also demands to protect the application of ADFP in preparation of kits for detecting or co-detecting whether a patient is a HBV-HCC patient or not. The invention also protects a kit for screening HBV-HCC patients, and the kit comprises a substance for detecting ADFP. The invention also protects a kit for detecting or co-detecting whether a patient is a HBV-HCC patient or not, and the kit comprises a substance for detecting ADFP. ADFP can be used to screen HBV-HCC patients so as to give an early warning, moreover, the result is precise, the operation is convenient, and thus the ADFP has a practical value.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
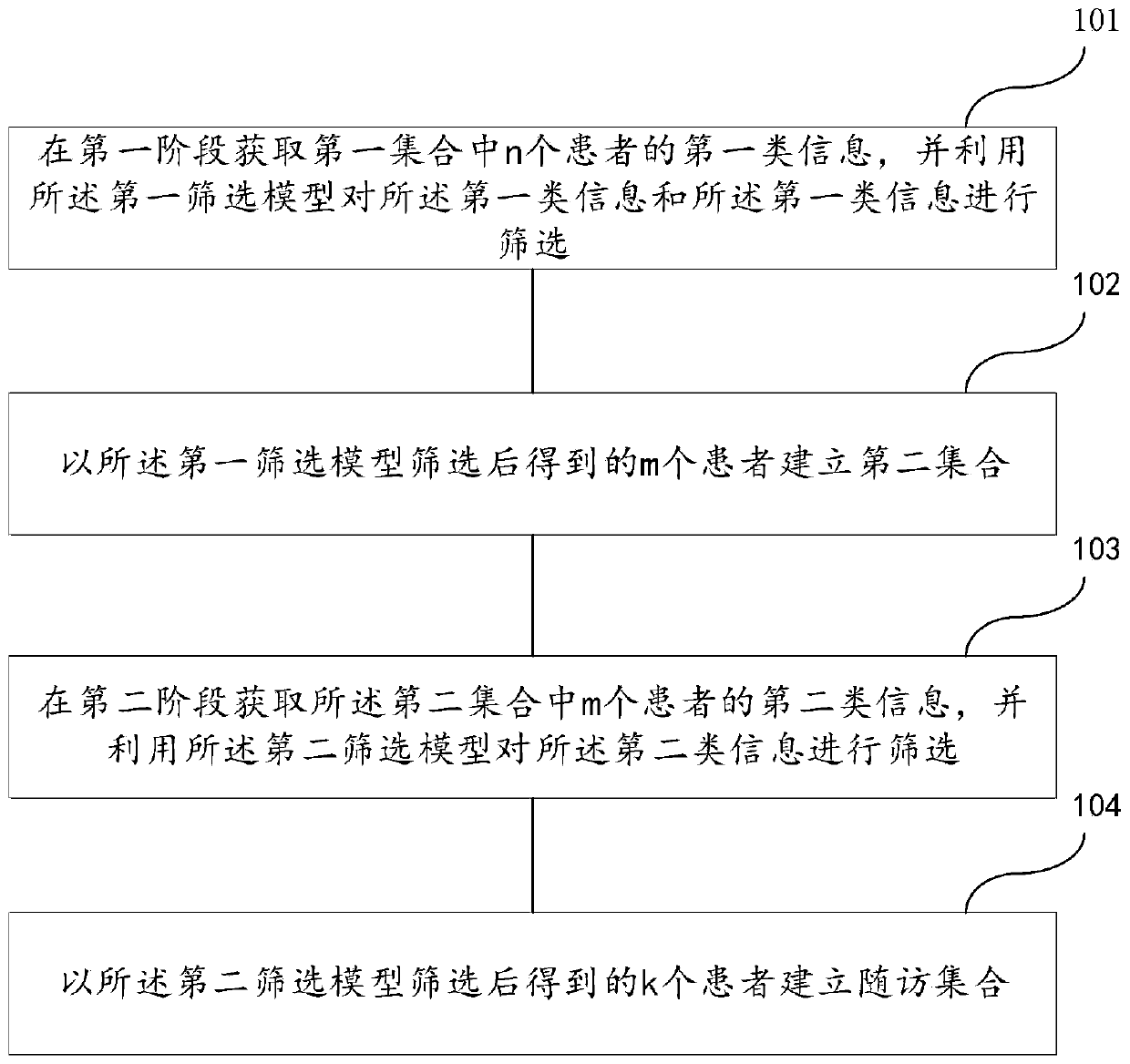
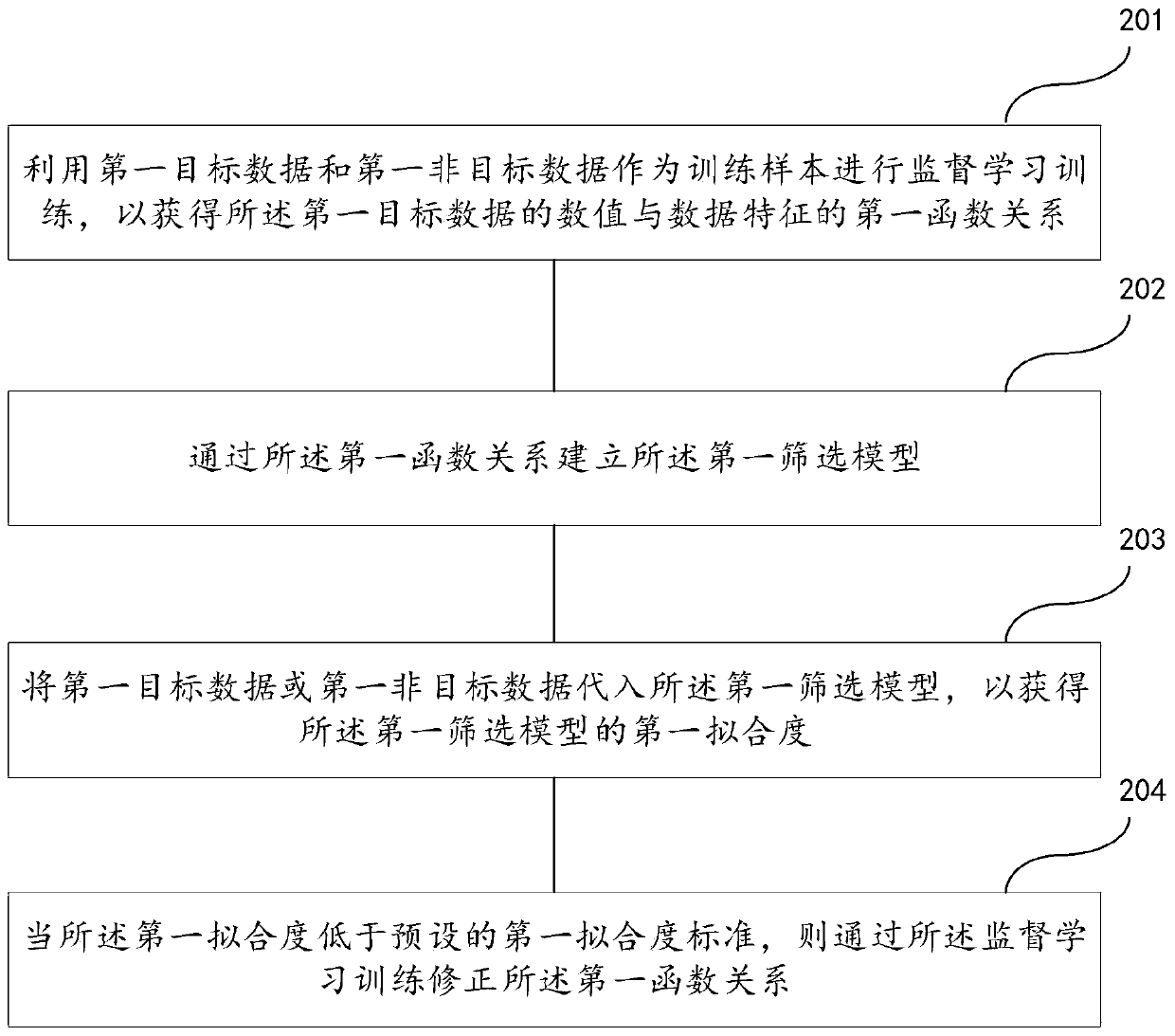
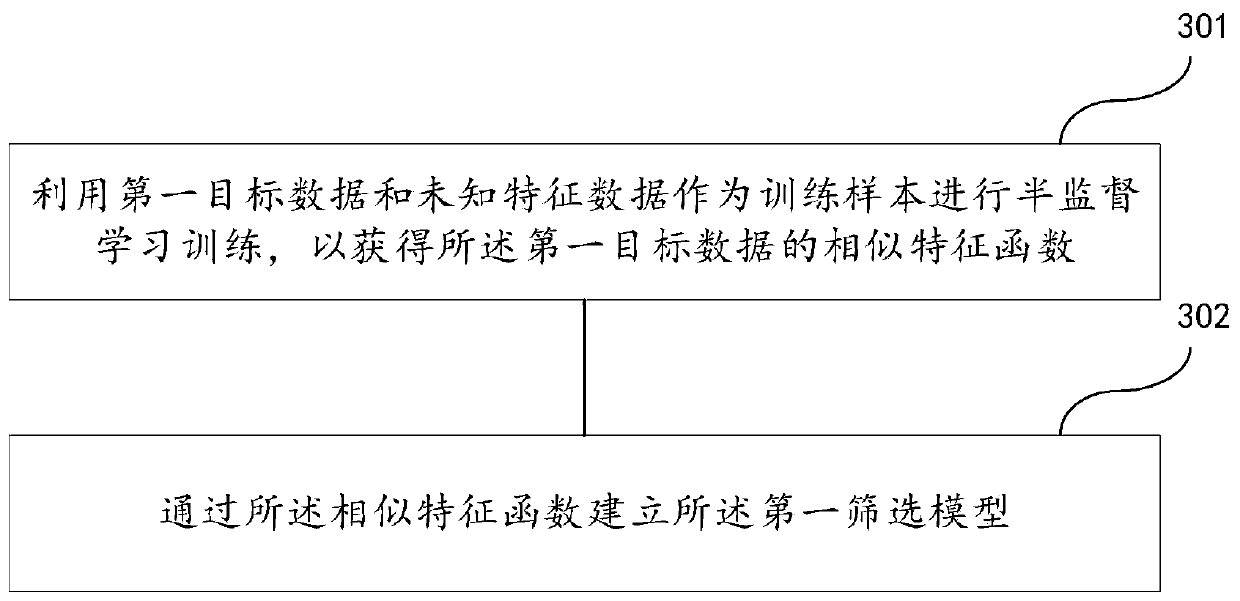
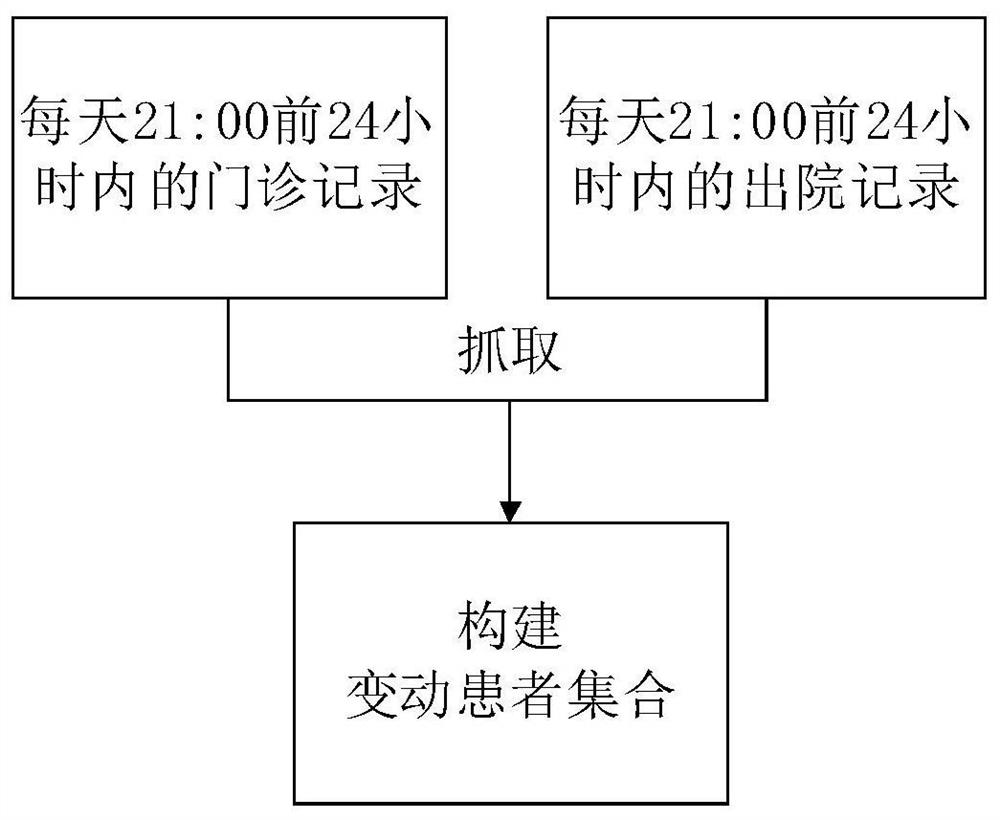
Follow-up surveying patient screening method, device, readable medium and electronic equipment
ActiveCN110400613AAvoid disturbanceImprove targetingCharacter and pattern recognitionPatient-specific dataScreening methodPhases of clinical research
The invention discloses a follow-up surveying patient screening method, a device, a readable medium and electronic equipment. The method comprises the steps of establishing a first screening model anda second screening model; acquiring first-class information of n patients in a first set in a first period, and screening the first-class information by means of a first screening model; establishinga second set according to m patients which are obtained through screening by means of the first screening model; acquiring second-class information of m patients in the second set in a second period,and screening the first-class information and the second-class information by means of a second screening model; and establishing a follow-up surveying set according to k patients which are obtainedthrough screening by means of the second screening model; wherein n, m and k are positive integers and n>=m>=k.
Owner:南京医基云医疗数据研究院有限公司
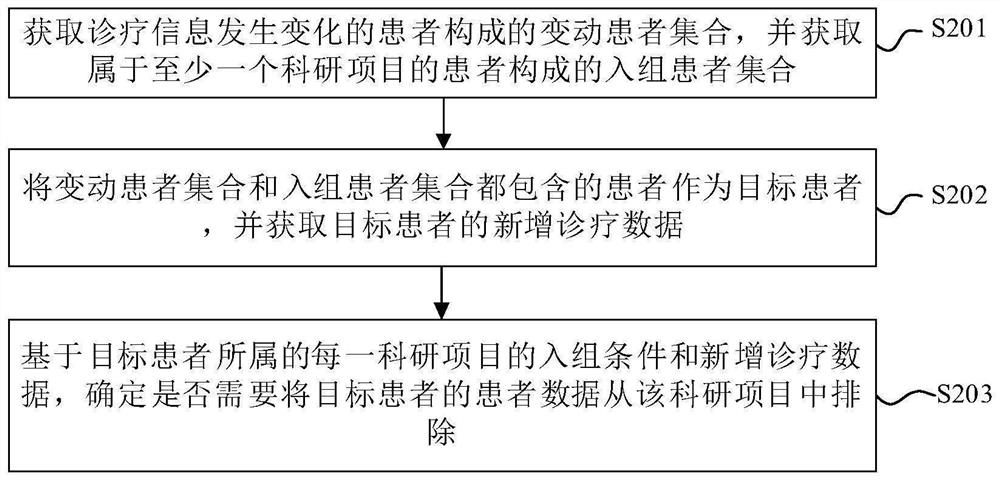
Clinical research patient screening method and corresponding device
PendingCN113889205AExclude in timeFast screeningMedical automated diagnosisPatient-specific dataPatient needClinical research
The embodiment of the invention provides a clinical research patient screening method and a corresponding device, and the method comprises the steps: obtaining a changing patient set composed of patients whose diagnosis and treatment information changes, and obtaining an in-group patient set composed of patients belonging to at least one scientific research project; taking patients contained in the changed patient set and the in-group patient set as target patients, and obtaining newly added diagnosis and treatment data of the target patients; and determining whether the patient data of the target patient needs to be excluded from the scientific research project or not based on the group entering condition of each scientific research project to which the target patient belongs and the newly added diagnosis and treatment data. According to the scheme, only part of data of part of patients need to be screened, compared with full-amount screening, the screening speed is higher, and the in-group patients not meeting the group entering requirement can be eliminated in time.
Owner:上海柯林布瑞信息技术有限公司
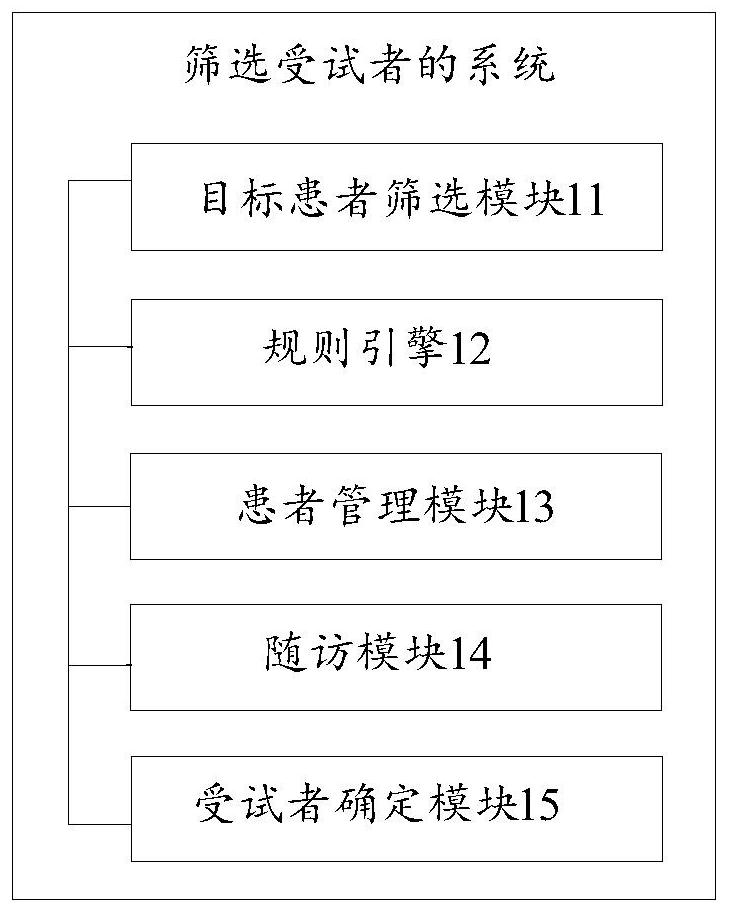
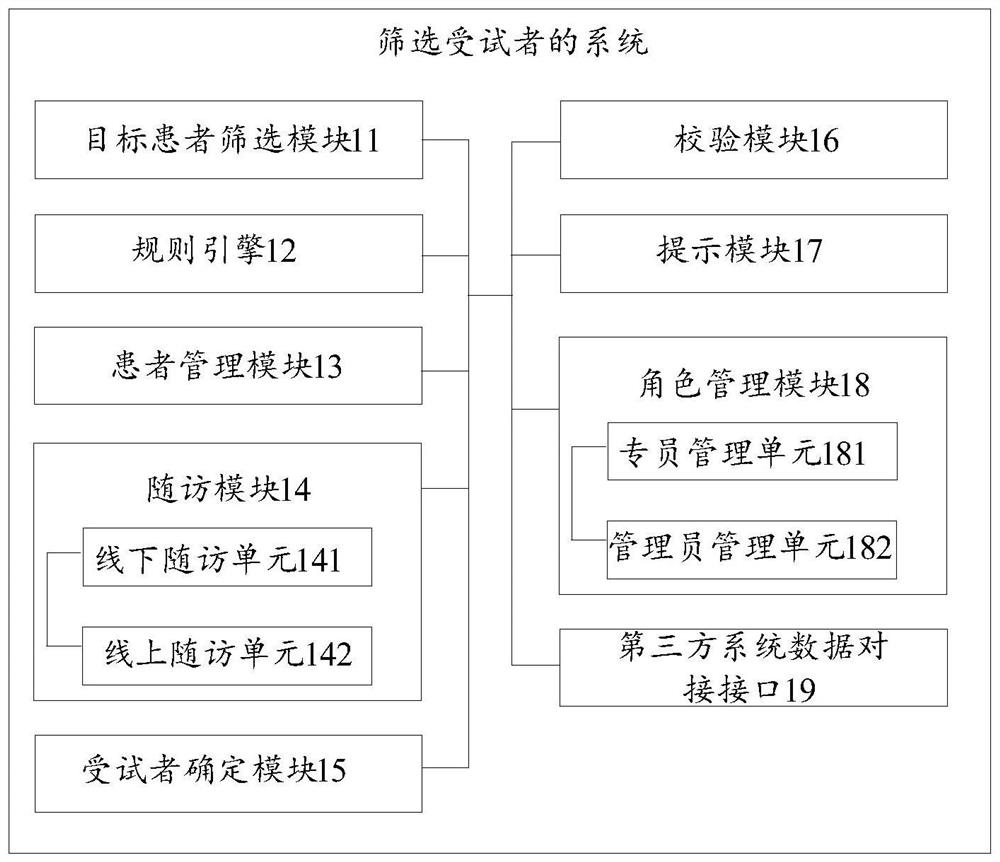
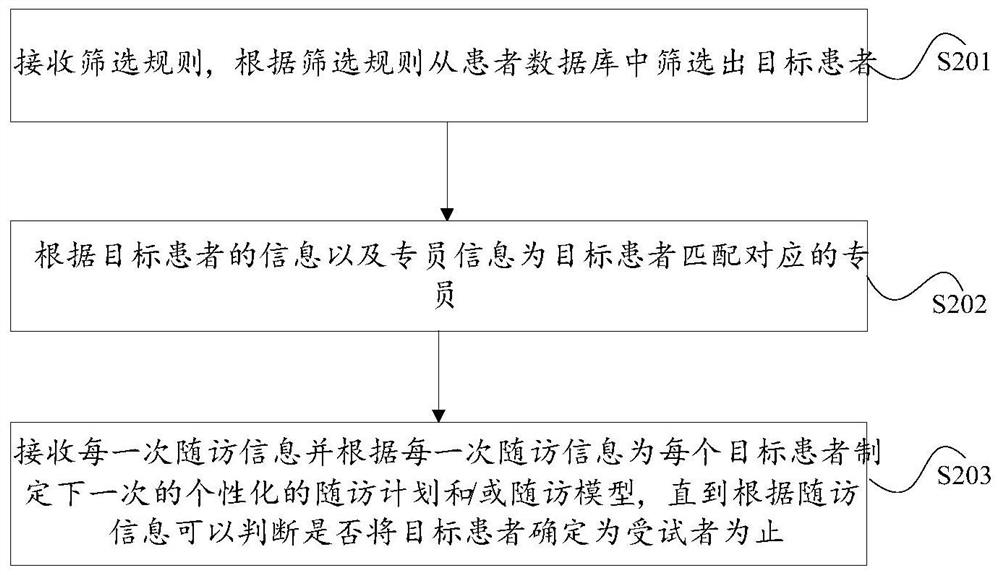
System and method for screening subjects
PendingCN112365940AGuaranteed accuracyEasy to trackDrug referencesPatient-specific dataMedical recordPharmacy medicine
The invention discloses a system and a method for screening subjects. The system comprises a target patient screening module used for automatically screening out target patients from a patient database according to a screening rule; a rule engine used for matching a corresponding specialist for the target patient according to the information of the target patient and the specialist information; apatient management module used for at least managing follow-up visit information and medical record information of the target patient; a follow-up visit module used for receiving follow-up visit information each time and making a next personalized follow-up visit plan and / or follow-up visit model for each target patient according to the follow-up visit information each time, wherein the follow-upvisit information is obtained according to the follow-up visit plan or the follow-up visit model; and a subject determination module used for judging whether the target patient is determined as a subject or not according to the follow-up visit information of each time. The invention aims to solve the problem of low efficiency of an existing method for determining drug subjects through manual screening.
Owner:LINKDOC TECH BEIJING CO LTD +1
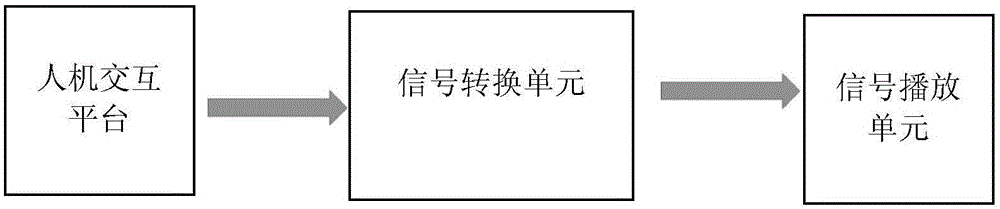
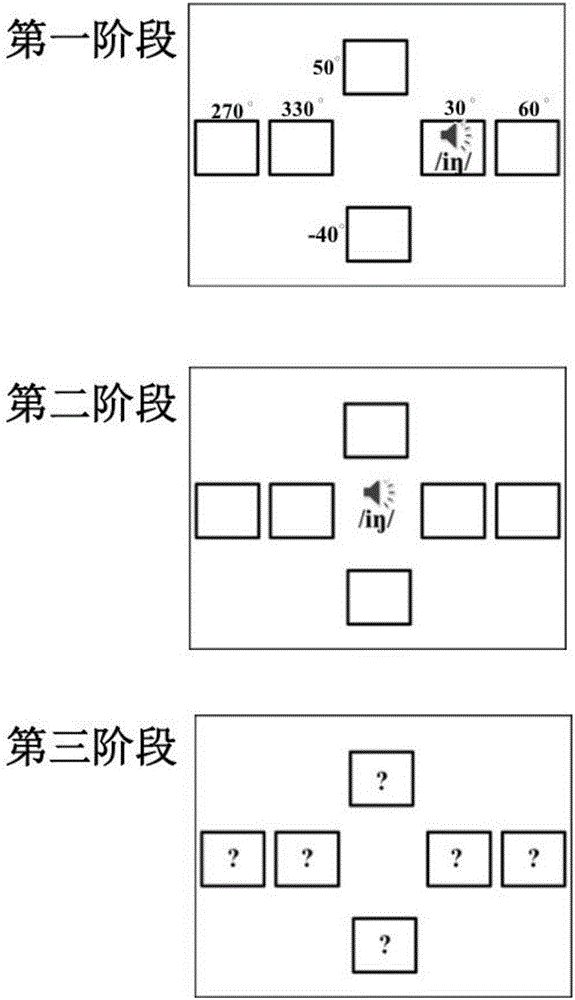
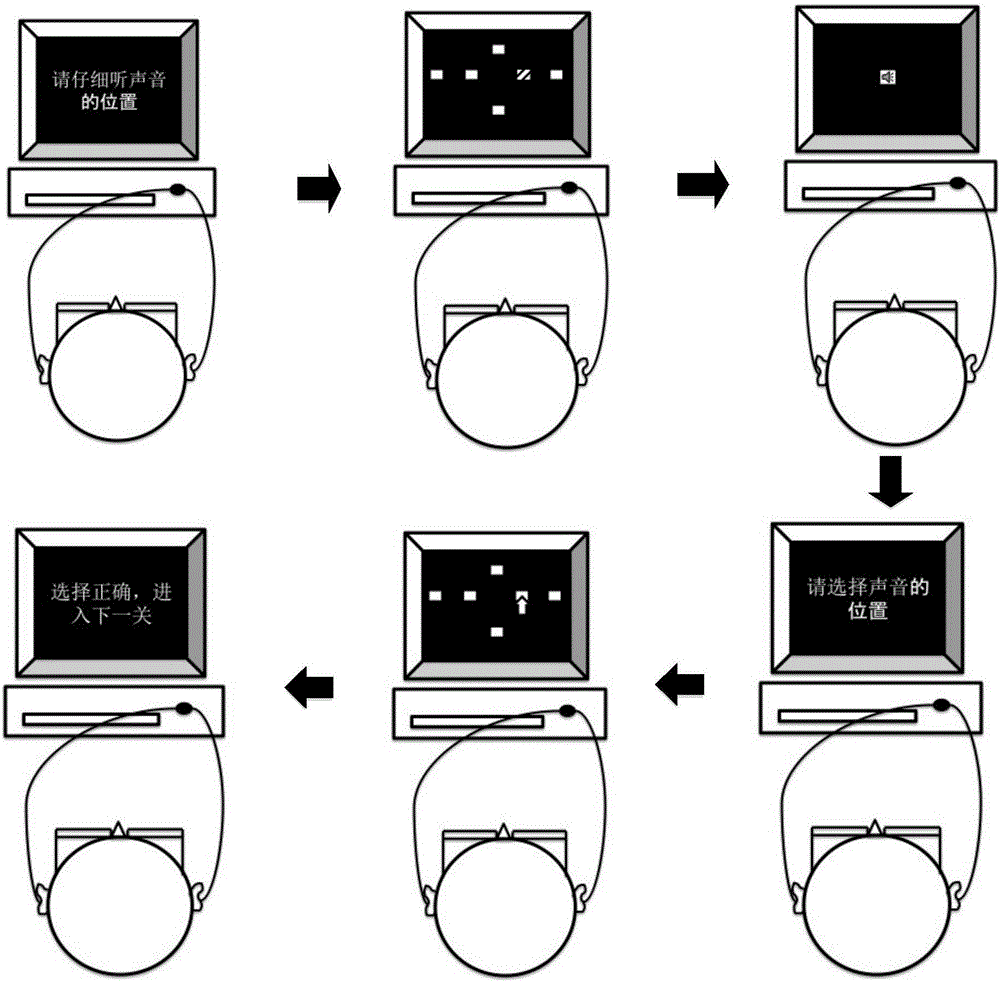
Alzheimer's disease patient screening system based on auditory-space matching method
The invention discloses an alzheimer's disease patient screening system based on an auditory-space matching system. The alzheimer's disease patient screening system is characterized by comprising a human-computer interaction platform, a signal conversion unit and a signal playing unit; the human-computer interaction platform is used for processing sound information selected through a head-related transfer function into a sound containing space information and sending the sound to the signal conversion unit; the signal conversion unit is used for converting the received sound data signal into an analog signal and sending the analog signal to the signal playing unit; the signal playing unit is used for playing the received sound analog signal. By means of the alzheimer's disease patient screening system, early-phase alzheimer's disease patients can be screened.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com