Immunogenic peptides of metalloprotease adamts-7 and its application in anti-atherosclerosis and related diseases
An arterial and protein technology, applied in the fields of biotechnology and medicine, can solve the problems of no effective short peptide vaccine reports, etc., achieve the effect of preventing or treating atherosclerosis and/or vascular restenosis, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, the preparation of ADAMTS-7 vaccine and the detection of titer
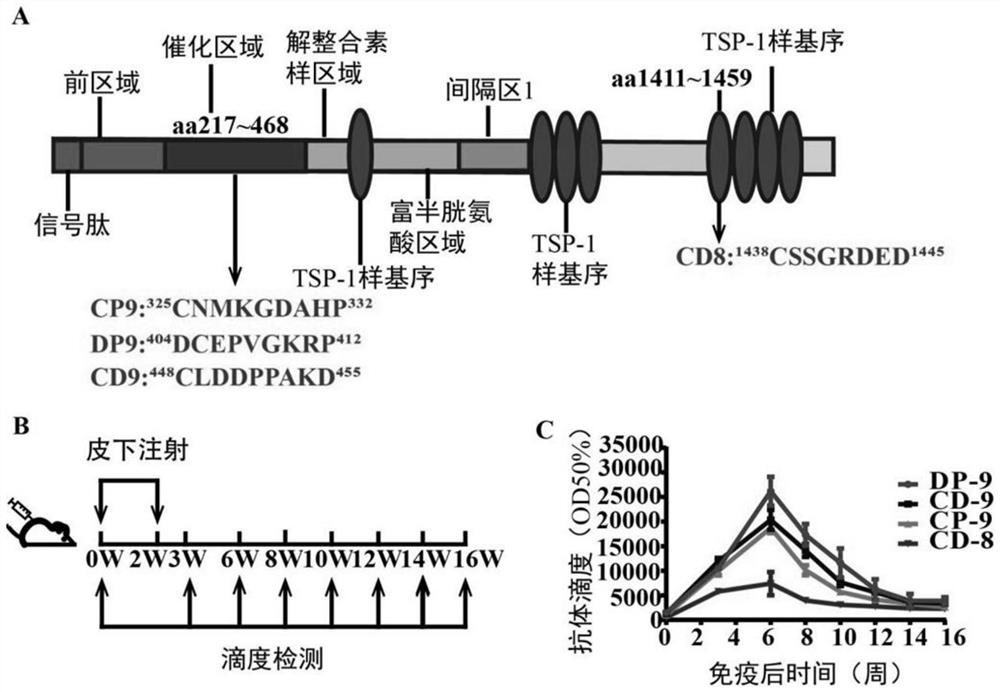
[0055] 1. Screening of immunogenic peptides of metalloprotease ADAMTS-7
[0056] ADAMTS7 mainly promotes VSMC migration and angiogenesis intima formation by binding and degrading COMP. The present invention selects catalytic domain and 4TSP-1 like domain as target regions for screening short peptides. Enter the amino acid sequences of the catalytic domain and the 4 TSP-1like domain into the dialog box of IEDB B Cell EpitopePrediction respectively, and according to the predicted score, select 8, 9, 10, 11, and 12 amino acids in units of 8, 9, 10, 11, and 12 amino acids in the higher score For short peptides with a high overall score, input the amino acid sequences of the above two regions into the IEDB dialog box at the same time, select the existing Crystal Structure Of Adamts4 With InhibitorBound with the highest similarity to ADAMTS7 as the model for epitope prediction, and combine the two ...
Embodiment 2
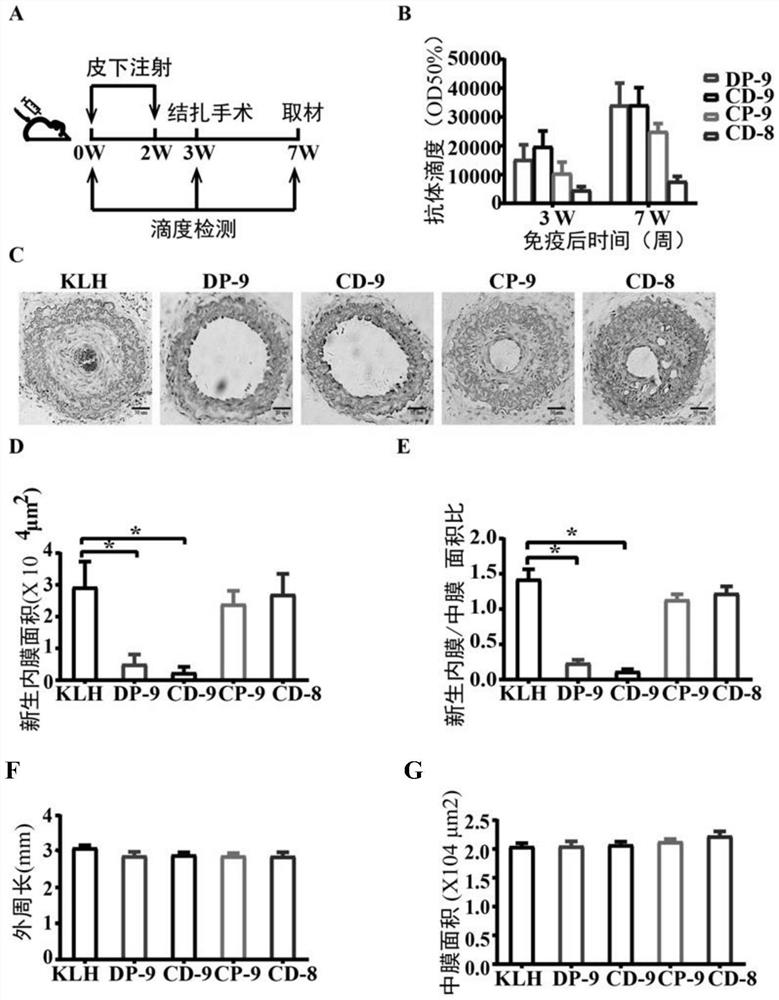
[0067] Example 2, Vaccine DP-9 and Vaccine CD-9 Inhibit Neointimal Formation Caused by Ligation Model
[0068] 1. Establishment of the total ligation model of the left neck of mice after immunization
[0069] The experimental mice were male 6-week-old C57BL / 6 mice (purchased from the Department of Animals, Peking University Health Science Center), a total of 40 mice, weighing 21-23g, were randomly divided into 5 groups, 8 mice in each group, respectively experimental group 1, experimental Group 2, experimental group 3, experimental group 4 and control group.
[0070] The four kinds of dry powders of conjugates (KLH-CP9, KLH-DP9, KLH-CD9, KLH-CD8) obtained in step 2 of Example 1 were dissolved in sterile saline respectively, and the four concentrations were all 1 mg / mL the conjugate solution. The 4 kinds of conjugate solutions were mixed with aluminum hydroxide adjuvant (purchased from HEART, product number BF040) at a volume ratio of 10:1 to obtain 4 kinds of vaccines, namel...
Embodiment 3
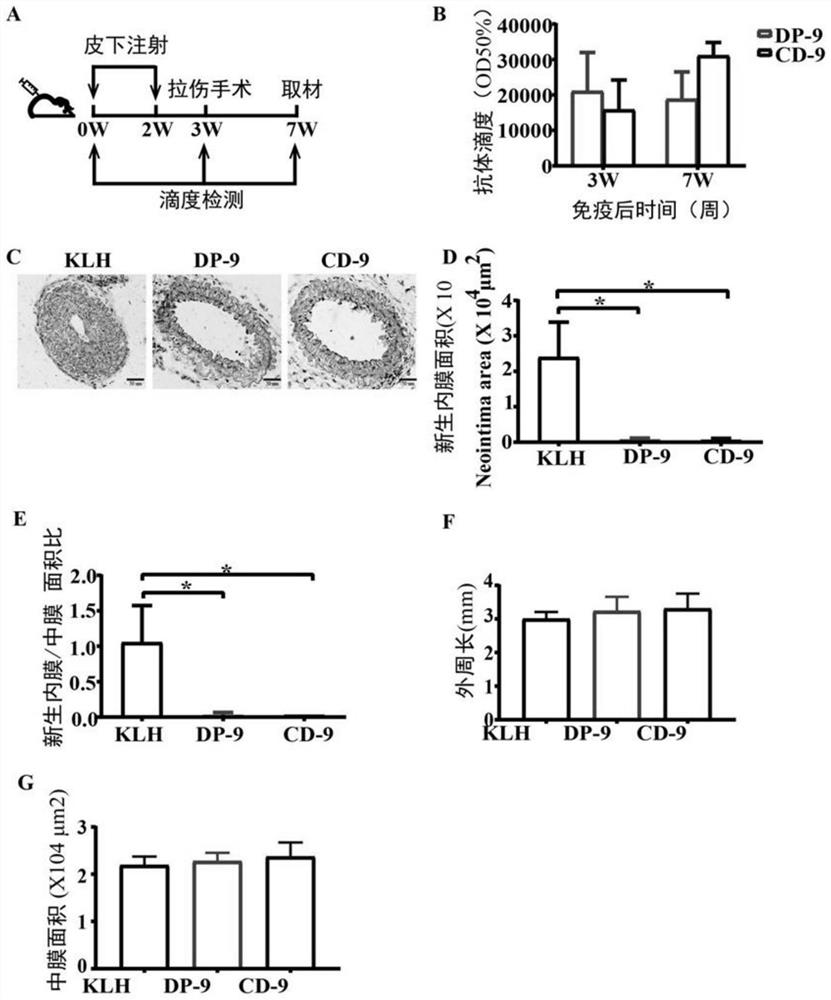
[0073] Example 3, Vaccine DP-9 and Vaccine CD-9 Inhibit the Neointimal Formation Caused by the Guide Wire Strain Injury Model
[0074]1. Establishment of mouse wire strain injury model after immunization
[0075] The experimental mice were male 6-week-old C57BL / 6 mice (purchased from the Animal Department of Peking University Health Science Center), a total of 30 mice, weighing 21-23g, were randomly divided into 5 groups, 6 mice in each group, respectively experimental group 1, experimental Group 2, experimental group 3, experimental group 4 and control group.
[0076] The four kinds of dry powders of conjugates (KLH-CP9, KLH-DP9, KLH-CD9, KLH-CD8) obtained in step 2 of Example 1 were dissolved in sterile saline respectively, and the four concentrations were all 1 mg / mL the conjugate solution. The 4 kinds of conjugate solutions were mixed with aluminum hydroxide adjuvant (purchased from HEART, product number BF040) at a volume ratio of 10:1 to obtain 4 kinds of vaccines, nam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com