Human ADAMTS-1 protein, gene encoding the same, pharmaceutical composition, and method for immunologically analyzing human ADAMTS-1 protein
a technology of human adamts and adamts family, which is applied in the field gene encoding the same, pharmaceutical composition, and immunological analysis of human adamts-1 protein, which can solve the problem that little is known about the physiological roles of these adam family proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of the Human ADAMTS-1 cDNA and Determination of the Base Sequence Thereof
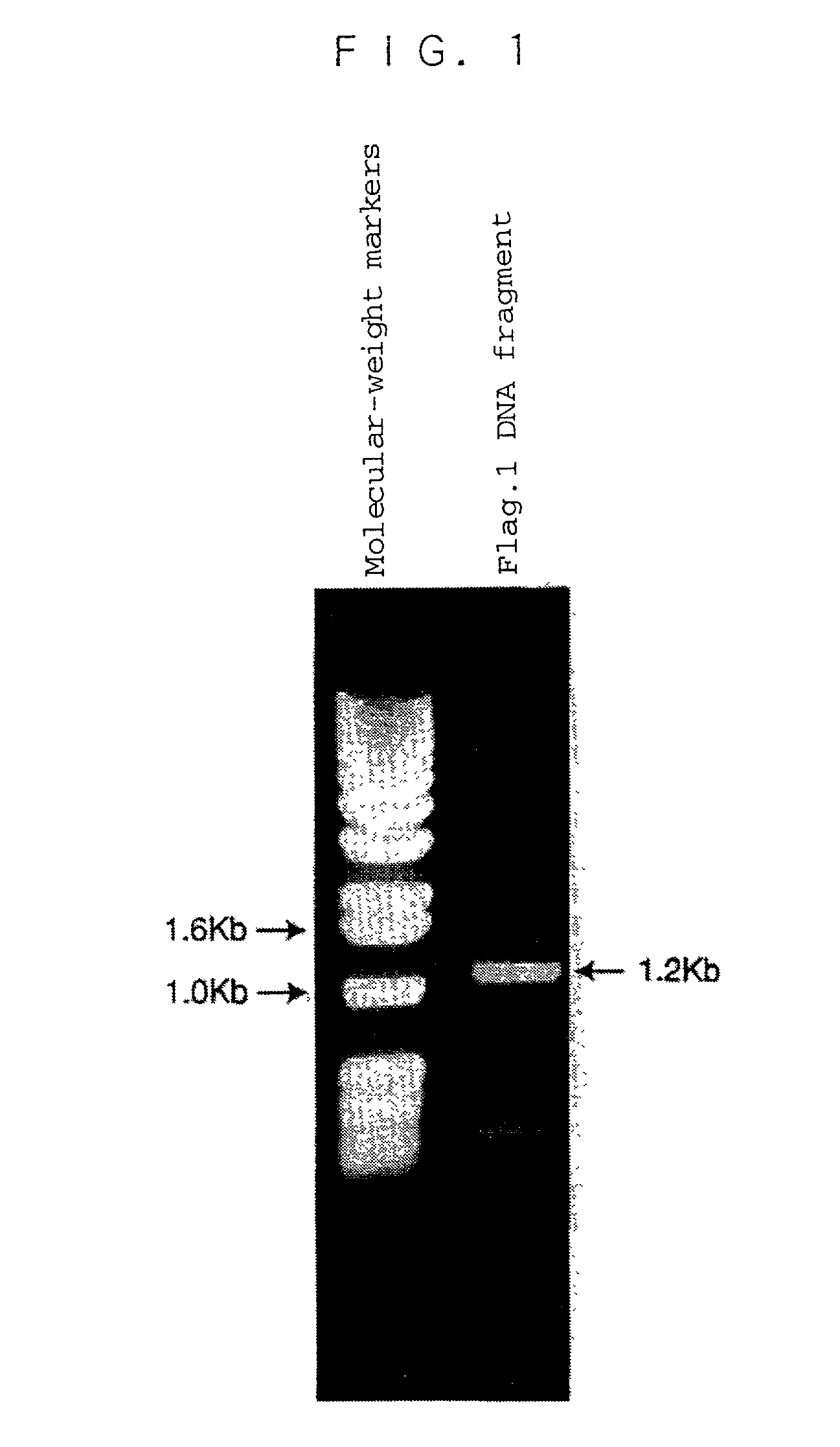
[0133] As primers for a PCR method, a DNA [hereinafter referred to as a "forward primer (1)"] having the base sequence (i.e., the base sequence of SEQ ID NO: 4: AGAACCTGTG GTGGTGGAGT TCAATACACA) corresponding to an amino acid sequence (i.e., the amino acid sequence of SEQ ID NO: 3: Arg Thr Cys Gly Gly Gly Val Gln Tyr Thr) contained in the first thrombospondin (TSP) domain from the N-terminus among three TSP domains of the mouse ADAMTS-1 protein [J. Biol. Chem., 272, 556-562 (1997)], and a DNA [hereinafter referred to as a "back primer (1)"] having the base sequence (i.e., the base sequence of SEQ ID NO: 5: CCTCTTAACT GCACTGTGTC AGTGTGCAAA AG) complementary to the base sequence encoding amino acids in the C-terminus of the mouse ADAMTS-1 protein and the base sequence in the vicinal regions (i.e., the regions upstream and downstream of the C-terminus) were chemically synthesized.
[0134] To 99 .mu.l of a ...
example 2
Preparation of the Human ADAMTS-1 Fusion Protein in E. coli
[0152] (1) Construction of an Expression Vector for E. coli
[0153] To introduce a SmaI site at the 5'-side and a NotI site at the 3'-side into the DNA encoding a partial region downstream of the MMP domain in the full-length human ADAMTS-1 protein, a forward primer (2) having the base sequence of SEQ ID NO: 10:
[0154] CACCCCGGGA GGAAGAAGCG ATTTGTGTCC AGCCCCCGTT ATG,
[0155] and a back primer (2) having the base sequence of SEQ ID NO: 11:
[0156] GTGGCGGCCG CCCTCTTAAC TGCACTGTGT CAGTGTGCAA AA
[0157] were chemically synthesized.
[0158] After 5 .mu.l of the forward primer (2), 5 .mu.l of the back primer (2), 1 .mu.l of a human kidney cDNA library (Marathon-Ready cDNA; Clontech Lab. Inc., Palo Alto, Calif., USA), 1 .mu.l of a Taq polymerase (0.5 unit; Ex Taq polymerase), 1 .mu.l of an anti-Taq polymerase antibody (Taq Start Antibody; Clontech Lab. Inc., Palo Alto, Calif., USA), 10 .mu.l of a PCR buffer having a 10-fold concentration (Cl...
example 3
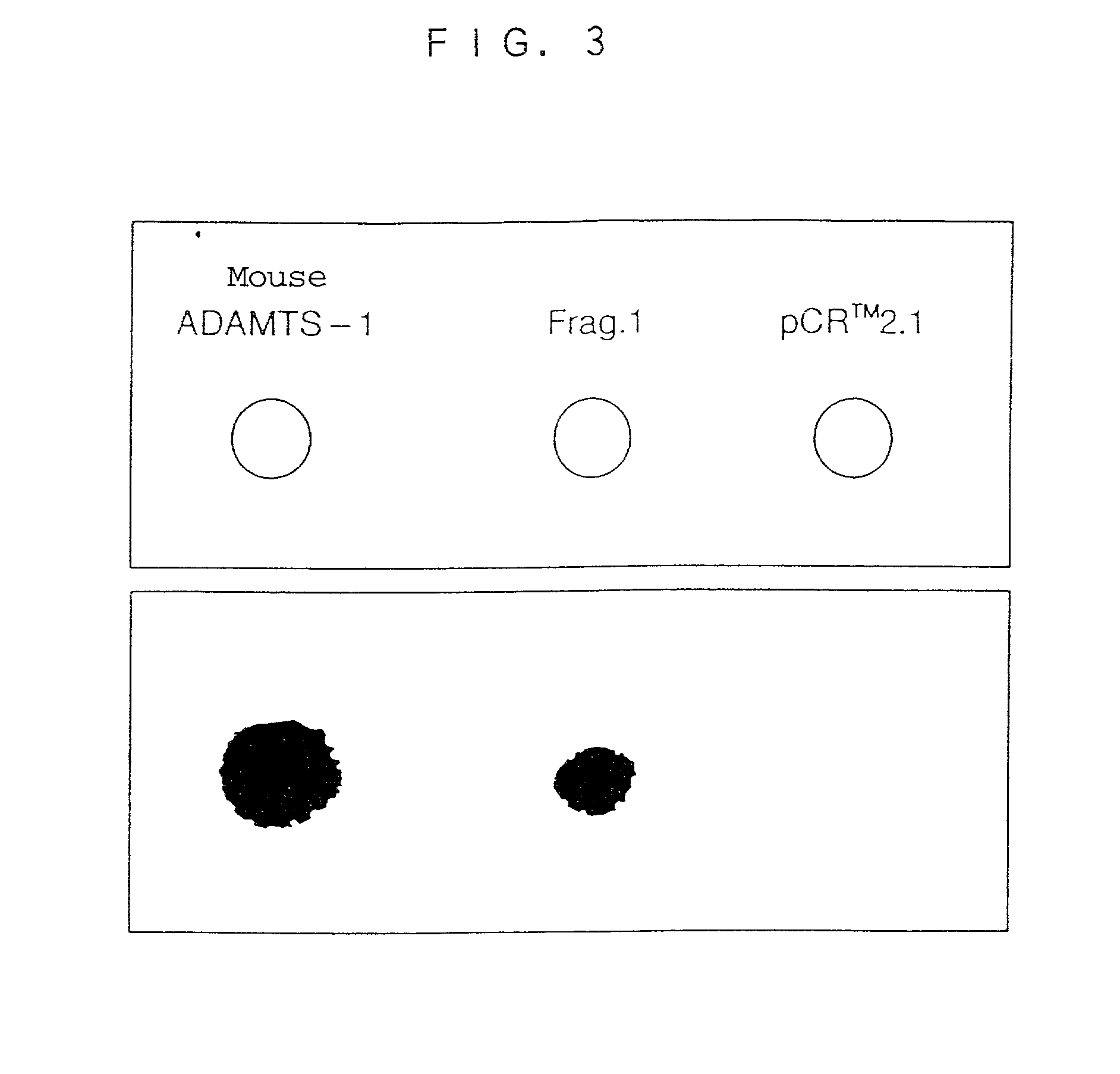
Examination of Activities of GST-Human ADAMTS-1 Fusion Protein on Influencing Hematopoietic Functions
[0172] To examine the activities of the GST-human ADAMTS-1 fusion protein on influencing hematopoietic functions, a large-scale preparation of the GST-human ADAMTS-1 fusion protein was carried out in accordance with the process disclosed in Example 2 (3), and about 30 .mu.g of the desired protein was obtained from 3 liters of E. coli culture.
[0173] The functions thought to influence the number of blood cells by a single dosage of the GST-human ADAMTS-1 fusion protein to a tail vein of a mouse were examined, as the activities influencing hematopoietic functions. The examining system can be conducted with a small amount of a protein to be examined, and enables a quick elucidation of a biological activity. In a control test, a GST protein extracted and purified by the process disclosed in Example 2(3) from E. coli transformed with a vector pGEX-5X-1 was used.
[0174] The GST-human ADAMTS-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com