ADAMTS-8 proteases and uses thereof
A protein and protease technology, applied in the field of disease treatment, can solve problems such as unidentified protease activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
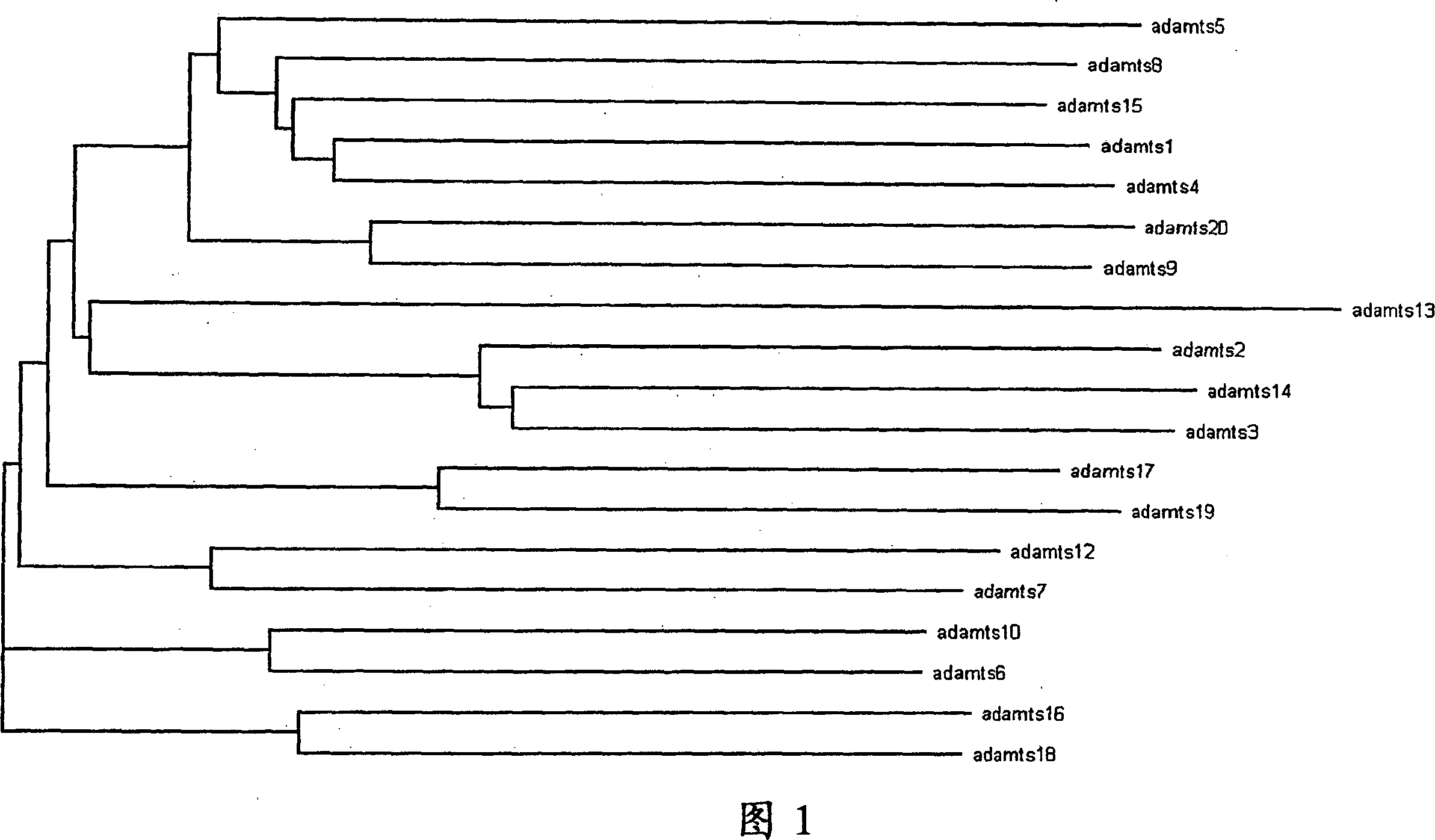
[0113] Example 1. Creating a Phylogenetic Diagram
[0114] Proteins from the following human ADAMTS family members were pooled to create a phylogenetic diagram: ADAMTS-1 / AB037767, ADAMTS-2 / AJ003125 (published sequences have the following changes from those used in the phylogenetic diagram: W643C, P1001L, and S1089C), ADAMTS-3 / AF247668, ADAMTS-4 / AF148213, ADAMTS-5 / AF142099, ADAMTS-6 / "SEQ ID NO: 2" in US Patent Application Publication 20020120113, ADAMTS-7 / AF140675, ADAMTS-8 / AF060153 (already The published sequence has the following changes compared to the sequence used in the phylogenetic map: L11P, F13L, L21P, P23Δ, L24Δ, and L129Q, where Δ represents a deletion), ADAMTS-9 / AF261918 (the published sequence and the sequence used in the phylogenetic map The sequence has the following changes compared to: G64S and S96T), "SEQ ID NO: 9" in ADAMTS-10 / PCT Publication No. WO 02 / 60942 (the published sequence has the following changes compared to the sequence used in the phylogenetic pl...
Embodiment 2
[0117] Example 2. Construction of ADAMTS-8 expression vector
[0118] The DNA sequence of ADAMTS-8 was deposited at GeneBank (Accession No. AF060153) by Vázquez et al., supra. To isolate the gene, 4 sets of oligonucleotide primer pairs spanning the ADAMTS-8 open reading frame were designed:
[0119] The first pair of primers included ATGTTCCCCGCCCCCGCCGCCCCCCGGTG (SEQ ID NO: 2) and GGATCCCCCGAGGCGCTCGATCTTGAACT (SEQ ID NO: 3). The second pair of primers included GGATCCGGCCGGGCGACCGGGGGC (SEQ ID NO: 4) and CTCTAGAAGCTCTGTGAGATACATGGCGCT (SEQ ID NO: 5). The third pair of primers included CTCTAGACGGCGGGCACGGAGACTGTCTCCTGGATGCCCCTGGTGCGGCCCTGCCCCTCCCCACA (SEQ ID NO: 6) and ACGTGTATTTGACTTTTGGGGGGAAGACCTCGCCAGGGACTGTCAGGAGCTGCACTGTCAGAGGCTC (SEQ ID NO: 7). The fourth pair of primers included CACACGTTCTTTGTTCCTAATGACGTGGACTTTAG (SEQ ID NO: 8) and GCGGCCGCTCACAGGGGGCACAGCTGGCTTTC (SEQ ID NO: 9).
[0120] The PCR amplification of adult lung cDNA library was carried out according to...
Embodiment 3
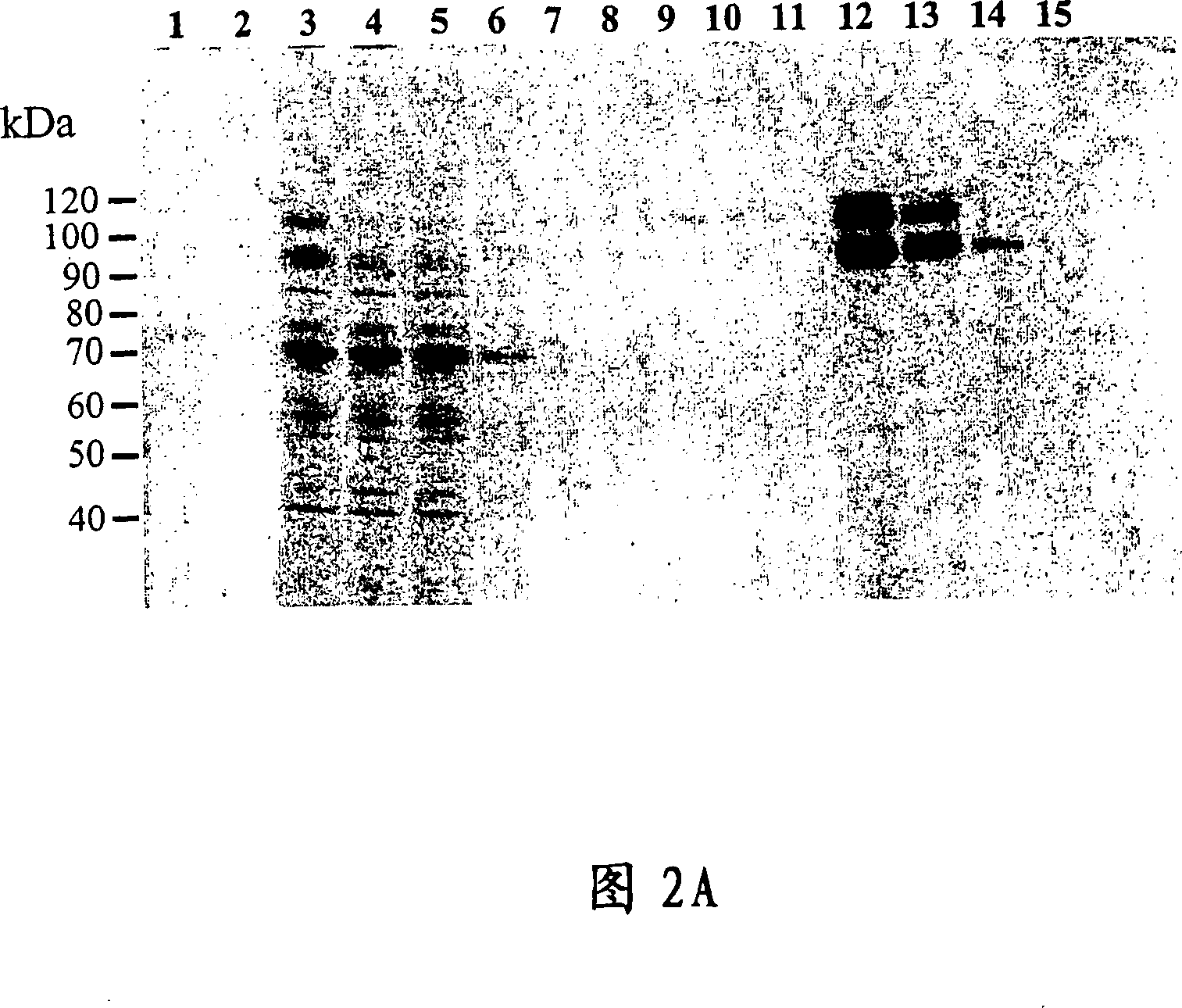
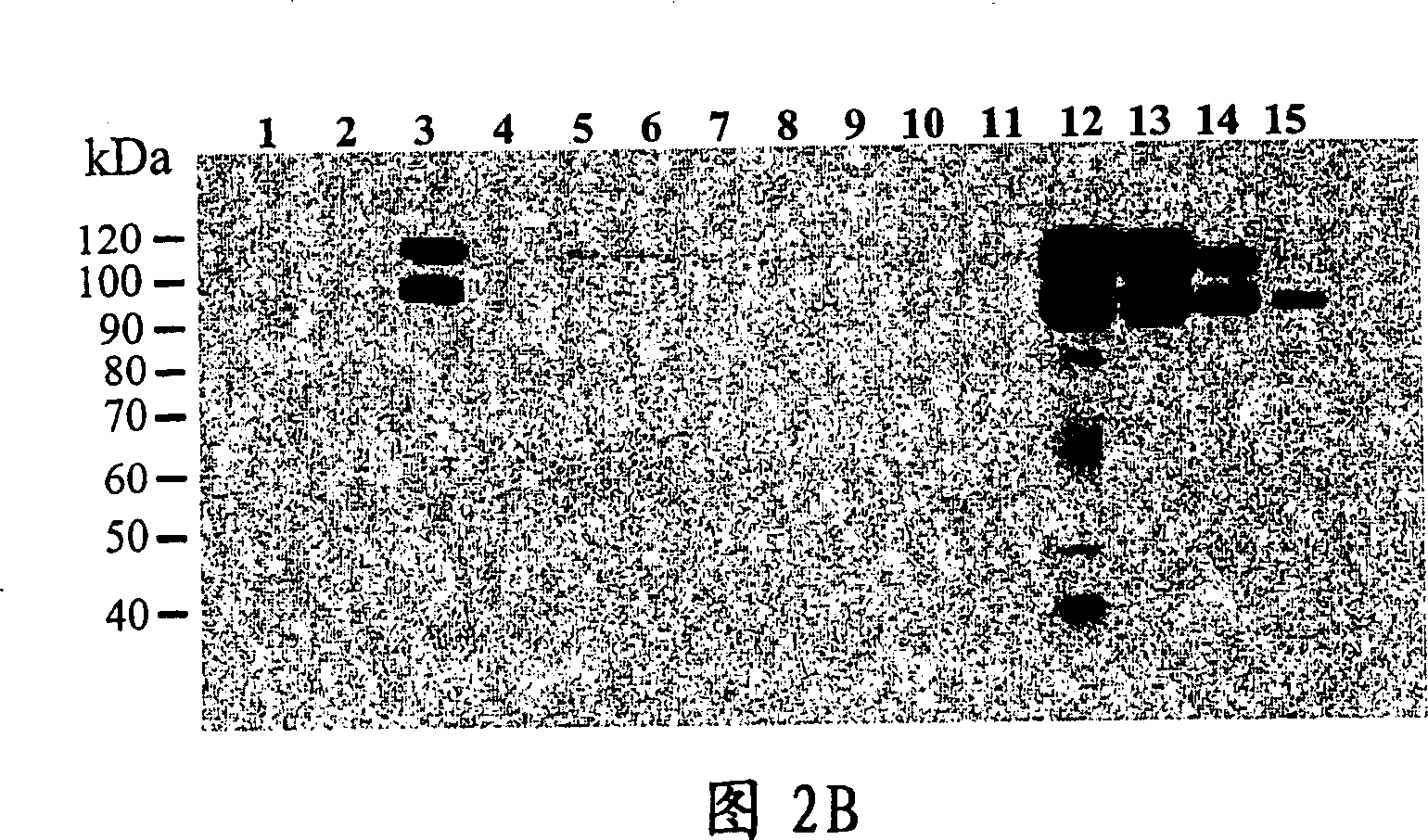
[0123] Example 3. Establishment of CHO cell lines expressing ADAMTS-8
[0124] CHO / A2 cells were used to establish a cell line stably expressing ADAMTS-8. The CHO / A2 cell line was derived from CHO DUKX B11 by stably integrating the transcriptional activator tTA, a fusion protein comprising the Tet repressor and the herpesvirus VP16 transcriptional domain. The ADAMTS-8 / pHTop expression vector contains six tet operator repeats upstream of the ADAMTS-8 sequence. In pHTop, tTA binds to the Tet operator to activate downstream gene transcription. A gene encoding dihydrofolate reductase can also be included in the pHTop expression vector, allowing selection of stable transfectants by viral methotrexate resistance. A CHO cell line expressing ADAMTS-8 extracellularly can also be established by transfecting pHTop / ADAMTS-8 DNA into CHO / A2 cells using the manufacturer's recommended lipofection (Lipofectin from InVitrogen) protocol. Clones were selected with 0.02 μM methotrexate. Cell ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com