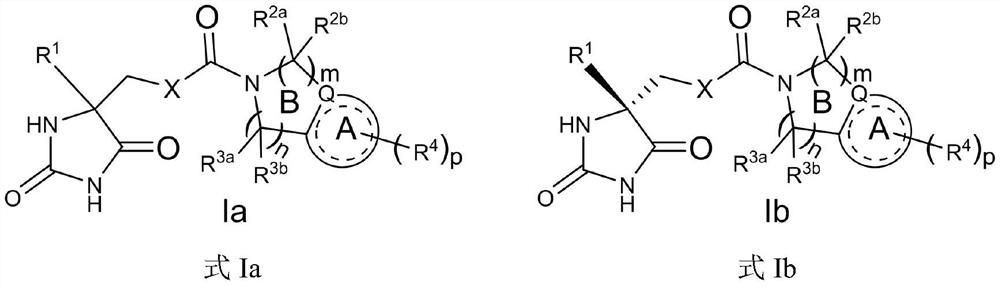
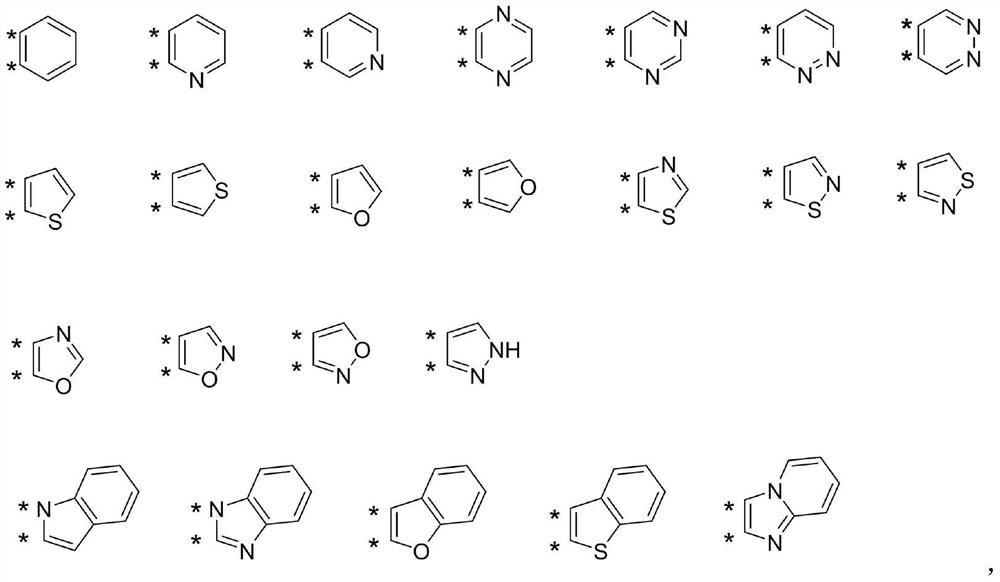
Condensed ring compound and preparation method and application thereof
A technology of compounds and oxides, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., can solve problems such as reducing the degradation of aggrecan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0252] In order to make the purpose and technical solution of the present invention clearer, the present invention will be further described below in conjunction with specific examples. It should be understood that these examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention. Moreover, the specific experimental methods not mentioned in the following examples are all carried out according to conventional experimental methods.
[0253] Abbreviations in this document have the following meanings:
[0254] Abbreviation meaning
[0255] TLC thin layer chromatography
[0256] LC-MS liquid chromatography-mass spectrometry
[0257] DMF N,N-Dimethylformamide
[0258] DMSO dimethyl sulfoxide
[0259] EA ethyl acetate
[0261] THF Tetrahydrofuran
[0262] LDA lithium diisopropylamide
[0263] LiHMDS lithium bistrimethylsilylamide
[0264] NaHMDS lithium bistrimethylsilylamide
[026...
Embodiment 1
[0320] Example 1: (S)-5-cyclopropyl-5-[3-(1,2,3,4-tetrahydroisoquinolin-2-yl)-3-oxopropane]imidazoline-2, Preparation of 4-diketones
[0321]
[0322] 3-[(4S)-4-cyclopropyl-2,5-dioximidazolidin-4-yl]propionic acid (70mg, 0.33mmol), EDCI (76mg, 0.396mmol), HOBT (54mg, 0.396mmol ) and DIPEA (128mg, 0.99mmol) were dissolved in 2mL of dichloromethane, stirred at room temperature for half an hour, then added 1,2,3,4-tetrahydroisoquinoline (44mg, 0.33mmol), and continued to stir for 12 hours. After the completion of the reaction monitored by TLC, 22 mg of the target compound (white solid, yield 20.4%) was separated by preparative TLC.
[0323] 1 H NMR (400MHz, DMSO-d 6 )δ:10.63(s,1H),7.74(s,1H),7.20-7.18(m,4H),4.61-4.59(m,2H),3.63-3.59(m,2H),2.88-2.85(t, J=6.0Hz, 1H), 2.77-2.74(t, J=6.0Hz, 1H), 2.47-2.43(m, 1H), 2.36-2.28(m, 1H), 2.03-1.91(m, 2H), 1.14 -1.04(m,1H),0.46-0.44(m,1H),0.35-0.28(m,2H),0.13-0.07(m,1H). ESI-MS(m / z)328.1[M+H] + .
[0324] Compounds shown in Table ...
Embodiment 18
[0334] Example 18: (S)-5-cyclopropyl-5-(3-(2-(3,5-difluorophenyl)-2,6-dihydropyrrolo[3,4-c]pyrazole Preparation of -5(4H)-yl)-3-oxopropyl)imidazoline-2,4-dione
[0335]
[0336] step 1 : Preparation of tert-butyl 2-(3,5-difluorophenyl)-2,6-dihydropyrrolo[3,4-c]pyrazole-5(4H)-carboxylate (Int18-2)
[0337] To a solution of starting material Int18-1 (500mg, 2.39mmol) and 3,5-difluoroiodobenzene (574mg, 2.39mmol) in DMF (6mL) were added sequentially potassium phosphate (1.01g, 4.78mmol), phosphide Copper (92 mg, 0.48 mmol) and (1S,2S)-(+)-1,2-cyclohexanediamine (55 mg, 0.48 mmol). Under a nitrogen blanket, the reaction was stirred at 120°C for 18 hours. Subsequently, the reaction solution was diluted with 50 mL of water, and extracted with ethyl acetate (50 mL×2). The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and finally separated by silica gel column chromatography (EA:PE=15:85) to obtain 250 mg of the target compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com