Method for Determining Condition of Disseminated Intravascular Coagulation
a technology of disseminated intravascular coagulation and condition determination, which is applied in the direction of instruments, biochemical equipment and processes, material analysis, etc., can solve the problems of affecting the survival rate of patients, so as to reduce the mortality rate, improve the survival rate, and improve the effect of accuracy diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Measurements of Coagulation and Fibrinolysis Marker Levels in Patients with DIC and Patients with TTP
[0049]Samples of 3.8% citrated plasma were collected from patients with DIC (n=23) and patients with TTP (n=2). Marker levels except for a platelet count were measured with LPIA-NV7 (Mitsubishi Kagaku Iatron) using commercially available kits (LPIA series; Mitsubishi Kagaku Iatron). Samples of 3.2% citrated plasma were collected, and a platelet count was measured with KX-21 (Sysmex).
[0050]The results of the measurements are shown in Table 1. In Table 1, PAI-1 means a plasminogen activator inhibitor-1, D-D means D-dimer, Fbg means fibrinogen, FDP-P means plasma FDPs, PLT means platelets, and TAT means a thrombin / antithrombin III complex. The patients diagnosed with DIC included patients with a remarkably decreased platelet count, but these patients could not be distinguished from patients with TTP, based on only conventional coagulation and fibrinolysis marker levels.
TABLE 1PICPAI-1D-...
example 2
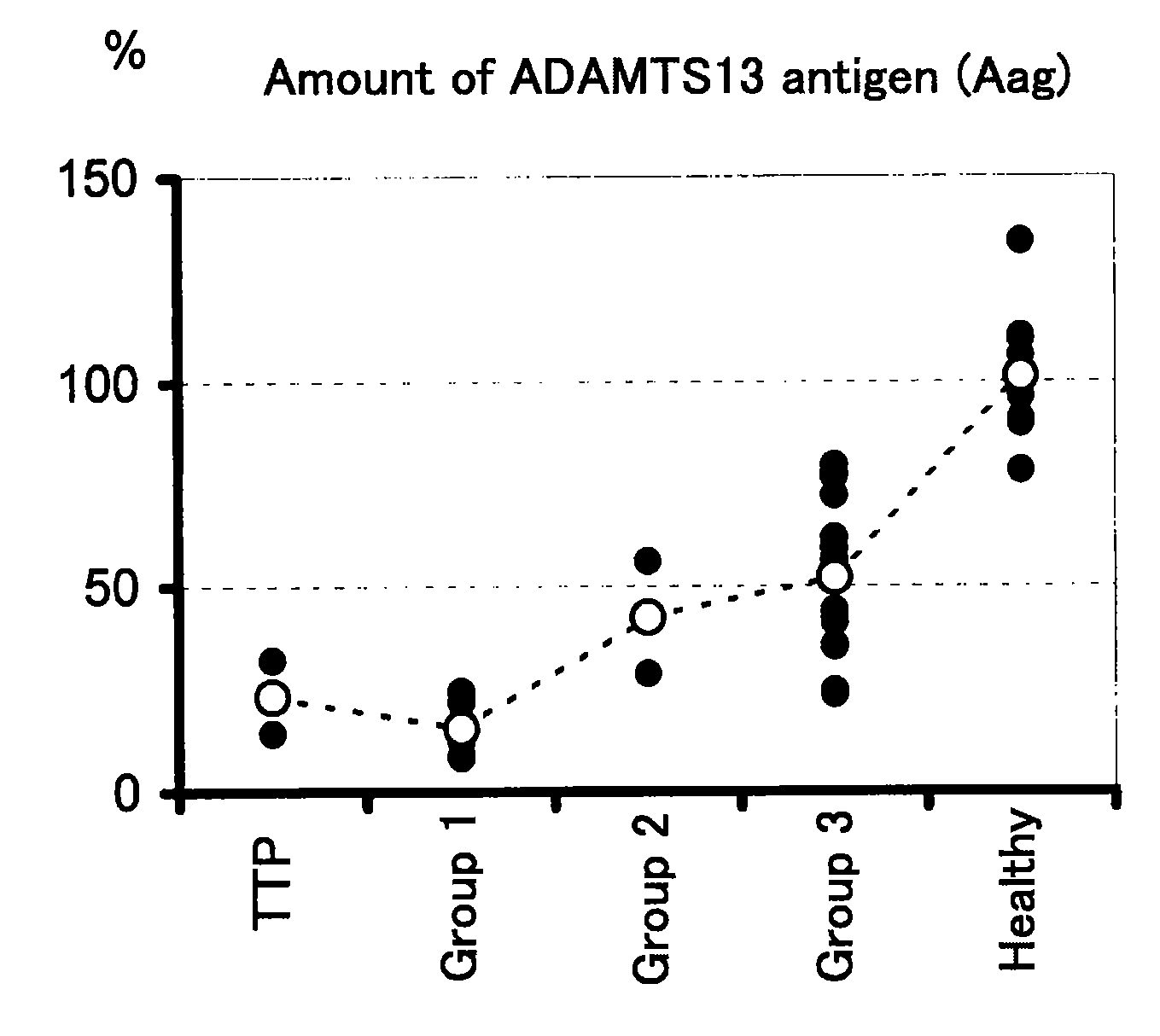
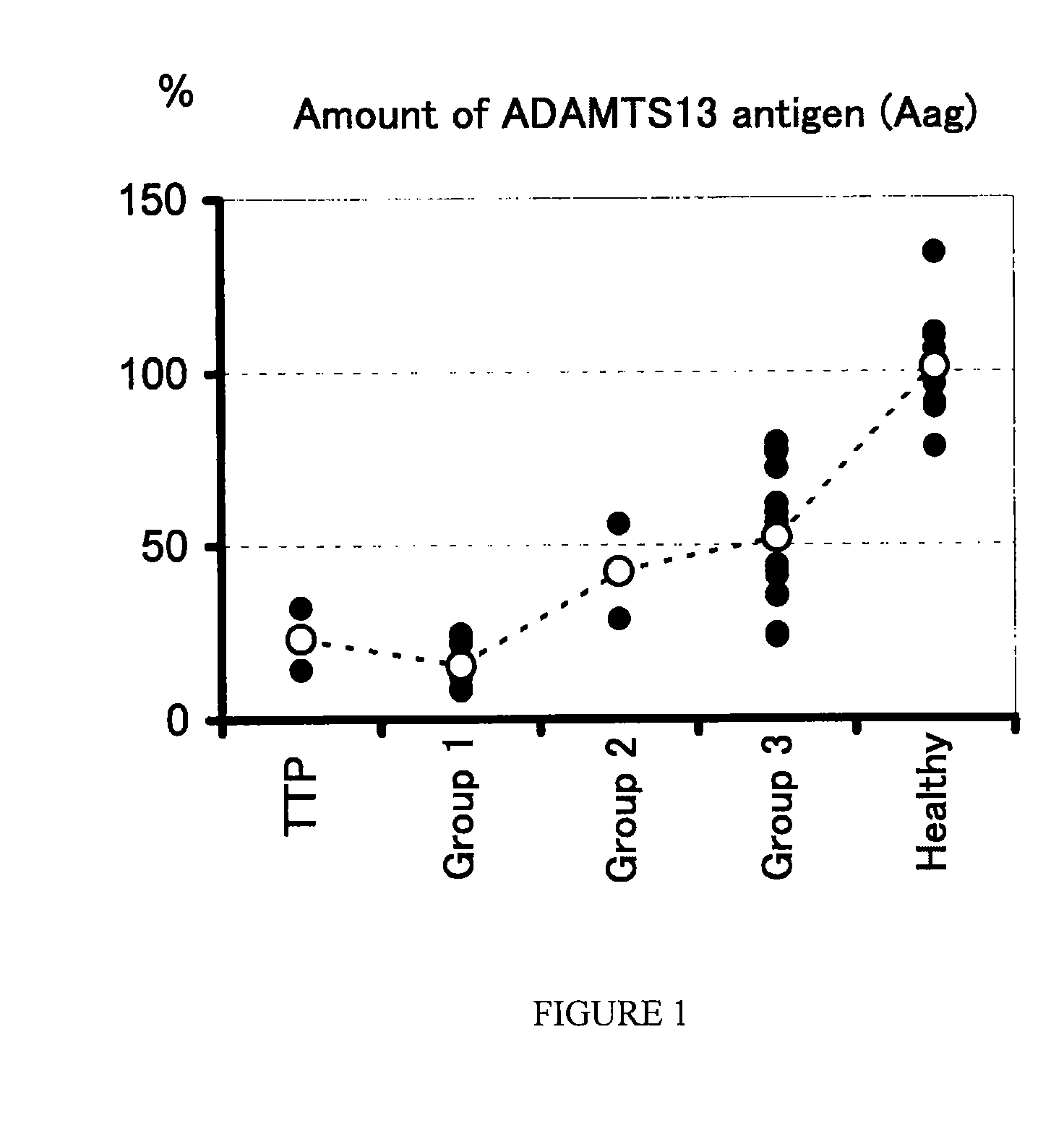
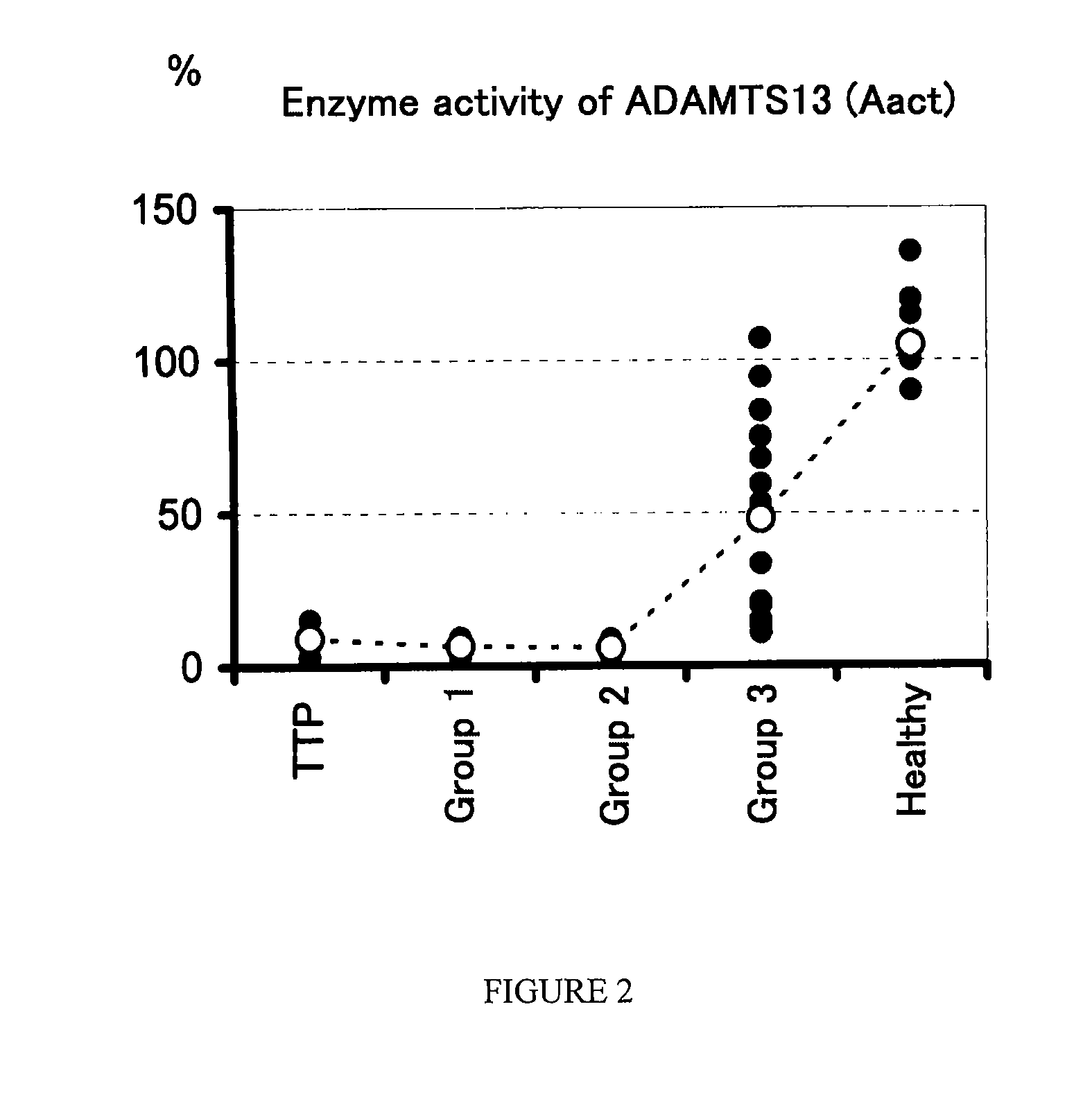
Analysis of Amount of ADAMTAS13 Antigen, Enzyme Activity of ADAMTS13, and Amount of vWF Antigen in Patients with DIC and Patients with TTP
[0051]Samples of 3.8% citrated plasma were collected from healthy persons (n=12), patients suffering from DIC (n=23) and patients suffering from TTP (n=2). In this regard, the DIC patients were diagnosed with DIC in accordance with the diagnostic criteria for DIC as described above, and the TTP patients were diagnosed with TTP in accordance with clinical findings. The amount (Aag) of an ADAMTS13 antigen was determined using a commercially available kit (ADAMTS-13 ELISA kit; Mitsubishi Kagaku Iatron). The enzyme activity (Aact) of ADAMTS13 was determined by an SDS-agarose gel electrophoresis [Furlan M. et al., Blood, (U.S.A.), 1997, vol. 89, p. 3097-3103]. The amount (Vag) of a vWF antigen was determined using a commercially available kit (STA LIAtest: Roche Diagnostics).
[0052]The results of the measurements are shown in Table 2. In Table 2, Aag an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enzyme activity | aaaaa | aaaaa |
| prothrombin time ratio | aaaaa | aaaaa |
| molecular-weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com