Preparation method of synaptic vesicle protein SV2A
A technology of proteins and vesicles, which is applied in peptide preparation methods, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of great differences in natural conformations, SV2A protein expressed in vitro, and lack of membrane proteins. Biological functions and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
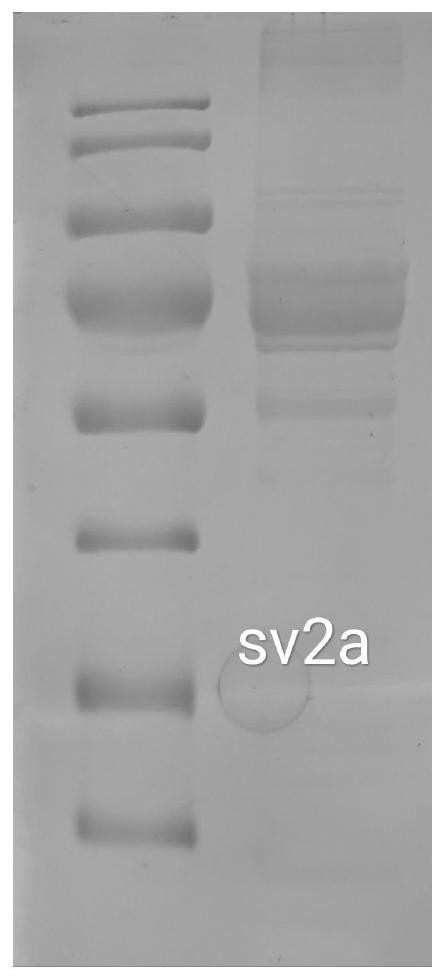
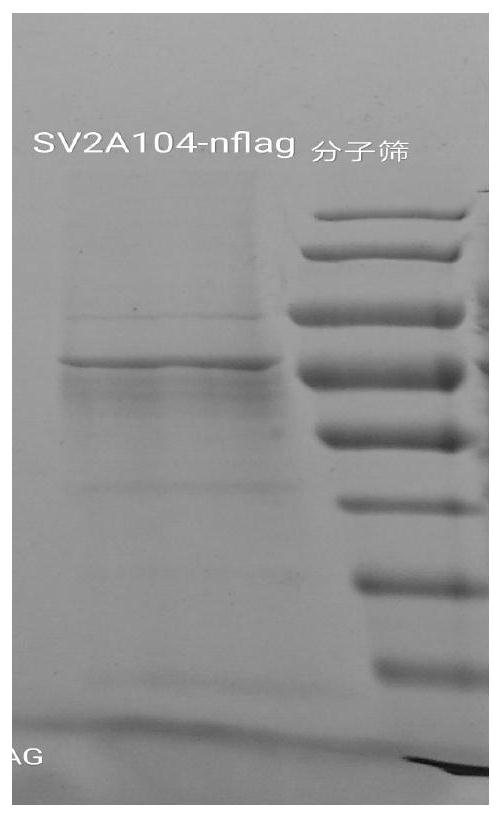
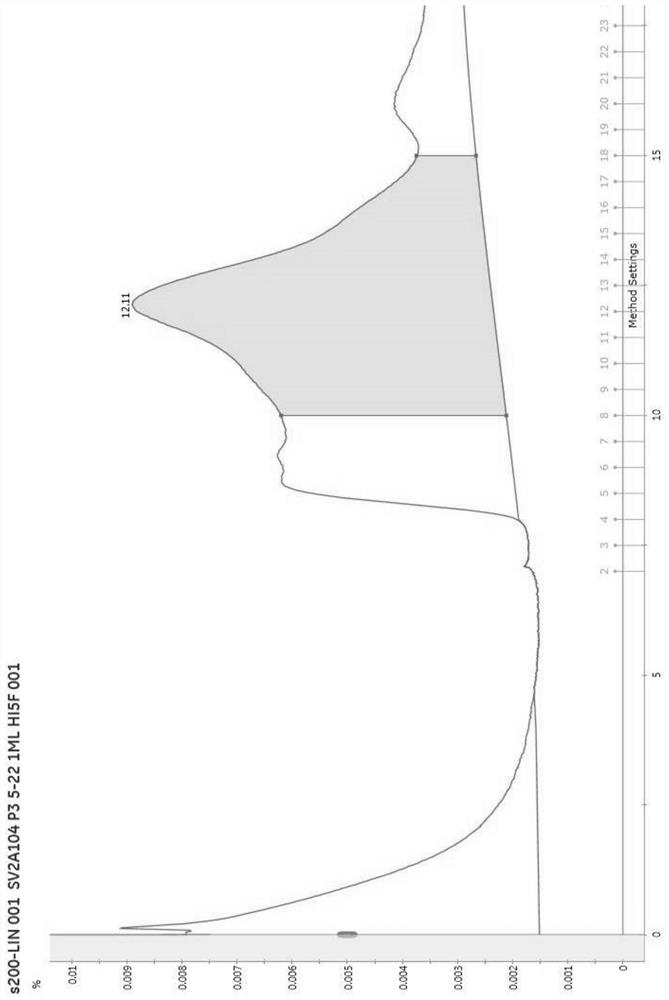
[0040] Embodiment 1 SV2A protein preparation method of the present invention
[0041] 1. Method
[0042] (1) Overexpression: construct the 104-742 base sequence of the human SV2A gene with N-flag tag (N-terminal flag tag) (the complete sequence of SV2A is shown in SEQ ID NO.1) on the Pfastbac vector , to transform DH10BAC bacteria to obtain Bacmid (baculovirus plasmid [a plasmid with a baculovirus genome that can shuttle between bacteria and insect cells]). Insect cell sf9 was transfected with Bacmid, and the virus produced was P1 generation, and then P2 and P3 generations were amplified, and P3 was used to infect high5 cells (Trichoplusia ni Hübnef egg cell line) or HEK293 cells, and the cells were collected 2 days after infection .
[0043] (2) Dissolution: Cells were collected by centrifugation at 600g for 10 minutes, and the cell liquid was discarded after collection. The cells were resuspended with 20mM Tris HCL150mM Nacl, and the protease inhibitor 1mM PMSF was added, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com