A kind of small molecule oxathiazide derivative and its application
A technology of oxathiazine and small molecules, which is applied in the field of small molecule oxthiazide derivatives, and can solve problems in the preclinical research stage or clinical trial stage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
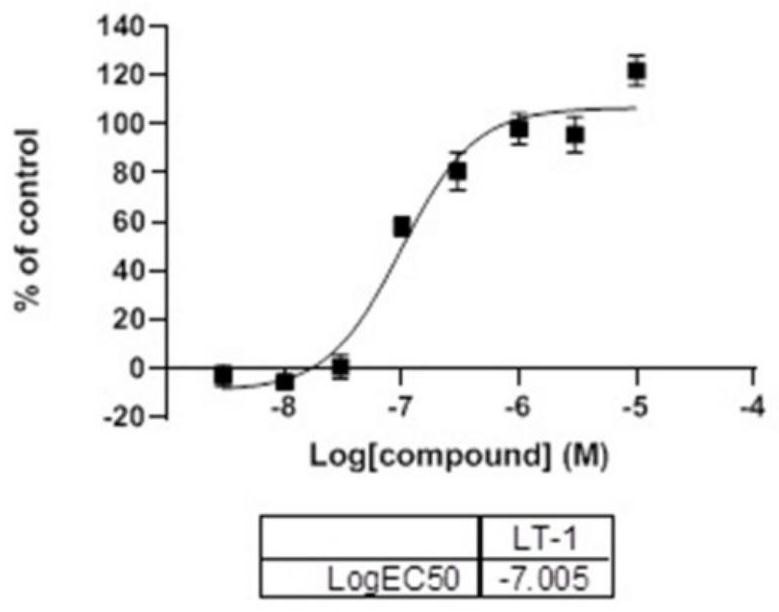
[0050] Compound disclosed in this example: LT-1,8-(4-methoxyphenyl)-3,4-dihydrobenzo[e][1,2,3]oxathiazine 2,2-dioxide (LT -1)
[0051]
[0052] Concrete synthetic route is as follows:
[0053]
[0054] The specific preparation method is as follows:
[0055] At 0°C, 3-bromo-2-hydroxybenzaldehyde (A01a) (5.6g, 28mmol) was dispersed in 60ml of dimethylacetamide DMA, and then aminosulfonyl chloride (10g, 86.5 mmol). The reaction system was stirred and slowly raised to room temperature until the reaction was complete. After the reaction, add water to precipitate the solid, filter and wash the filter cake with water and ethyl acetate respectively, and obtain the off-white compound as 8-bromobenzo[e][1,2,3]oxathiazine 2 with a yield of 85%. 2-dioxide (A01b).
[0056] Then A01b (2.62g, 10mmol) was dispersed in 50ml of MeOH solvent system, sodium borohydride (380mg, 10.6mmol) was added in batches at room temperature, and stirring was continued at room temperature after the add...
Embodiment 2
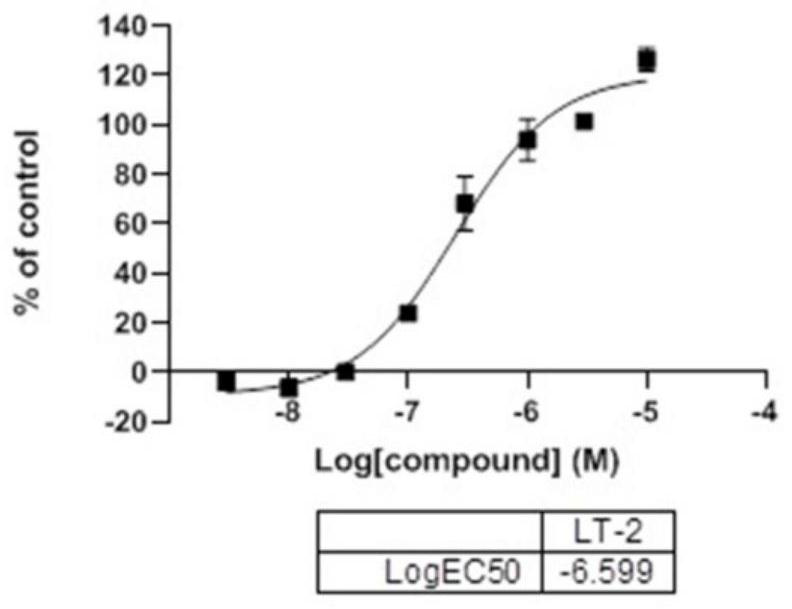
[0060] The compound disclosed in this example: 8-(4-methylphenyl)-3,4-dihydrobenzo[e][1,2,3]oxathiazine 2,2-dioxide (LT-2).
[0061]
[0062] The synthetic route is as in Example 1, replacing "p-methoxyphenylboronic acid" with "p-tolueneboronic acid".
[0063] MS(ESI)276.1[M+H] + ; 1 H NMR (400MHz, DMSO) δ8.54(s, 1H), 7.39–7.23(m, 7H), 4.62(s, 2H), 2.36(s, 3H).
Embodiment 3
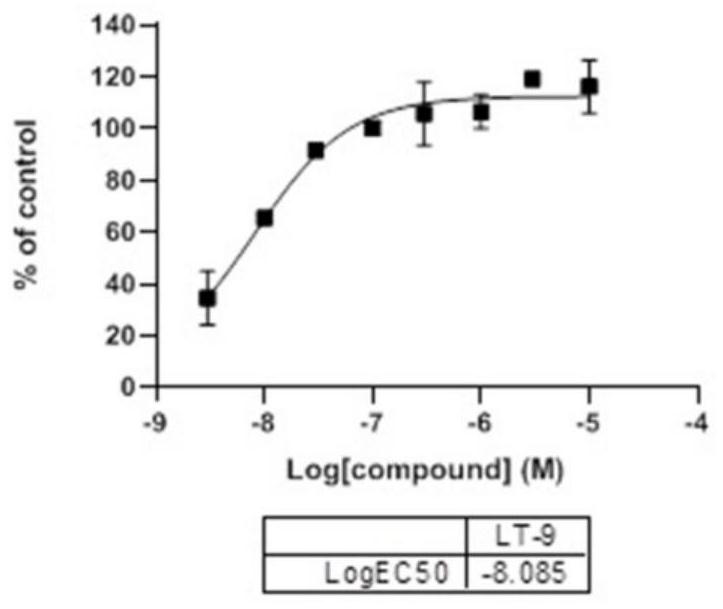
[0065] Compounds disclosed in this example: 8-(4-(tert-butyl)phenyl)-3,4-dihydrobenzo[e][1,2,3]oxathiazine 2,2-dioxide (LT- 3)
[0066]
[0067] The synthetic route is as in Example 1, replacing "p-methoxyphenylboronic acid" with "p-tert-butylphenylboronic acid".
[0068] MS(ESI)318.1[M+H] + ; 1 H NMR (400MHz, DMSO) δ8.56(s, 1H), 7.51(d, J=8.4Hz, 2H), 7.43–7.34(m, 3H), 7.33–7.21(m, 2H), 4.62(s, 2H), 1.33(s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com