Preparation method of juvenile hormone analogue
A juvenile hormone and analogue technology, applied in the field of preparation of juvenile hormone analogues, can solve the problems of low material yield and content, high sewage treatment cost, cumbersome operation steps, etc., and achieve shortened reaction cycle and easy operation , the effect of simple processing steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
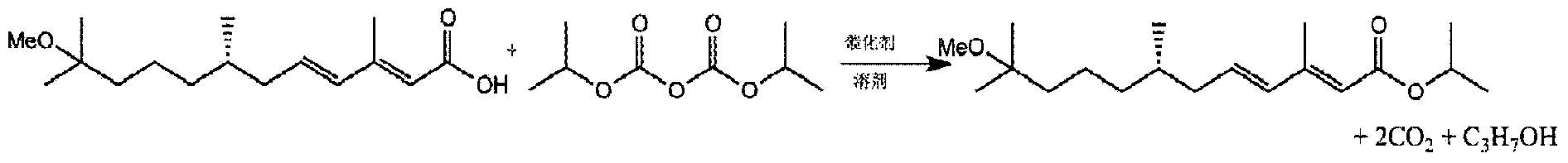
[0059] Prepare a dry and clean 1L four-necked reaction flask, and add (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoic acid to the flask sequentially 26.9g (0.1mol), catalyst 4-dimethylaminopyridine 1.22g (0.01mol) and toluene 100mL, stirred at 15-30°C; add diisopropyl pyrocarbonate toluene solution dropwise into the flask (diisopropyl pyrocarbonate ester 26.6g, 0.14mol; toluene 133mL); after the dropwise addition, stir and react at 15-30°C for 2h; add 23.3mL water into the flask, stir for 5min, let stand for phase separation, and separate the upper toluene layer; The organic phase was concentrated under pressure, and 27.9 g of S-methoprene product was obtained by distillation, with a weight yield of 103.7% and a molar yield of 90%. Product content: 97.3%, isomer: 0.8%.
Embodiment 2
[0061] Prepare a dry and clean 1L four-necked reaction flask, and add (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoic acid to the flask sequentially 26.9g (0.1mol), catalyst pyridine 9.1g (0.1mol) and dichloromethane 26.9mL, stir at 0-25°C; add diisopropyl pyrocarbonate dichloromethane solution (diisopropyl pyrocarbonate) dropwise into the flask Ester 38.0g, 0.2mol; dichloromethane 38mL); After the dropwise addition, add 64.9mL water in the flask, after stirring for 5min, let stand to separate the phases, separate the lower dichloromethane layer; concentrate the organic phase under reduced pressure, S-methoprene product was obtained by distillation 27.8g, with a weight yield of 103.3% and a molar yield of 90%. Product content: 97.4%, isomer: 0.8%.
Embodiment 3
[0063] Prepare a dry and clean 1L four-necked reaction flask, and add (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoic acid to the flask sequentially 26.9g (0.1mol), diisopropyl pyrocarbonate 26.6g (0.14mol) and toluene 269mL, stirred at 15-25°C; add the toluene solution of the catalyst 4-dimethylaminopyridine (4-dimethylaminopyridine) dropwise into the flask Aminopyridine 1.22g, 0.01mol; toluene 6.1mL); after the dropwise addition, stir and react at 15-30°C for 1h. Add 138mL of water into the flask, stir for 5min, let stand to separate the phases, and separate the upper toluene layer; concentrate the organic phase under reduced pressure, and distill to obtain 27.9g of S-methoprene product, with a weight yield of 103.7% and a molar yield of 90 %. Product content: 97.3%, isomer: 0.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com