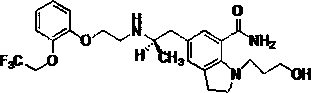
Silodosin film-coated tablet and preparation method thereof
A technology of silodosin tablets and silodosin, which is applied to the oral solid dosage form medicine for the treatment of benign prostatic hyperplasia, the field of silodosin, and can solve the problem of lack of physiological activity, substandard dissolution, tablet sticking and punching, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Excipient Compatibility Test
[0029] Mix 5 parts of mannitol, starch, pregelatinized starch, low-substituted hydroxypropyl cellulose with 1 part of silodosin, and then mix with 1 part of magnesium stearate. Store under storage conditions 1 and 2 respectively, observe the color change directly with the naked eye, and detect the degradation products by HPLC.
[0030] Storage conditions
[0031] Condition 1: 40°C, 75% relative humidity, 4 weeks
[0032] Condition 2: 40°C, 60% relative humidity, 4 weeks
[0033] Analytical method
[0034] Take an appropriate amount of mixed powder, add an appropriate amount of methanol, sonicate for 5 minutes to dissolve silodosin, cool, and dilute with [methanol-sodium chloride solution (1→200) (7:3)] to make about 0.2 mg silodosin solution, shake well, filter, and take the subsequent filtrate as the test solution.
[0035] Chromatographic conditions
[0036] Wavelength: 225nm
[0037] Column temperature: 40°C
[0038] Column: Age...
Embodiment 2
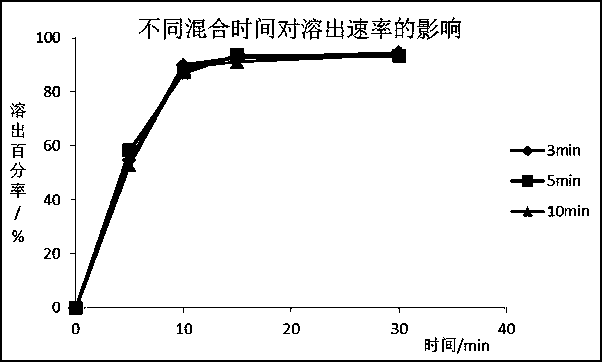
[0049] With pregelatinized starch, starch, and mannitol as fillers, low-substituted hydroxypropyl cellulose as disintegrant, and starch slurry as binder, silodosin tablets were prepared by wet granulation technology, and the prescription of the present invention was investigated. Electrostatic properties, compressibility, dissolution effects of silodosin.
[0050] Table 3 Prescription of Silodosin Tablets (grams)
[0051] formula
[0052] Preparation Process:
[0053] Grinding the raw material of silodosin until the particle size of D90 is 20-70 μm, weighing silodosin, pregelatinized starch and part of low-substituted hydroxypropyl cellulose respectively according to the weight ratio, mixing and wet granulating; The granulated product is dried and sized, added with additional excipients and magnesium stearate, mixed evenly, and compressed into tablets.
[0054] The content and content uniformity are used as the electrostatic detection index.
[0055] The hardness ...
Embodiment 3
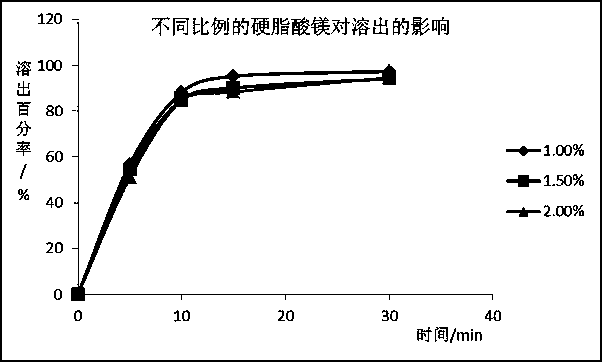
[0070] Influence of adding amount of magnesium stearate on dissolution rate of silodosin
[0071] Add 1%, 1.5%, and 2% magnesium stearate to fixed proportions of silodosin, mannitol, starch, pregelatinized starch, and low-substituted hydroxypropyl cellulose compositions, measure the dissolution rate, and investigate Effect of magnesium stearate addition on the dissolution rate of silodosin. Dissolution was determined according to the same method as described in Example 2.
[0072] Table 5 Formulation composition (grams)
[0073] formula
[0074] The preparation process is the same as in Example 2.
[0075] Table 6 Effect of different magnesium stearates on dissolution rate test results
[0076] formula
[0077] The results showed that the amount of magnesium stearate had almost no effect on the dissolution rate when pregelatinized starch was added to the prescription, which further explained that the addition of pregelatinized starch allowed the prescrip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com