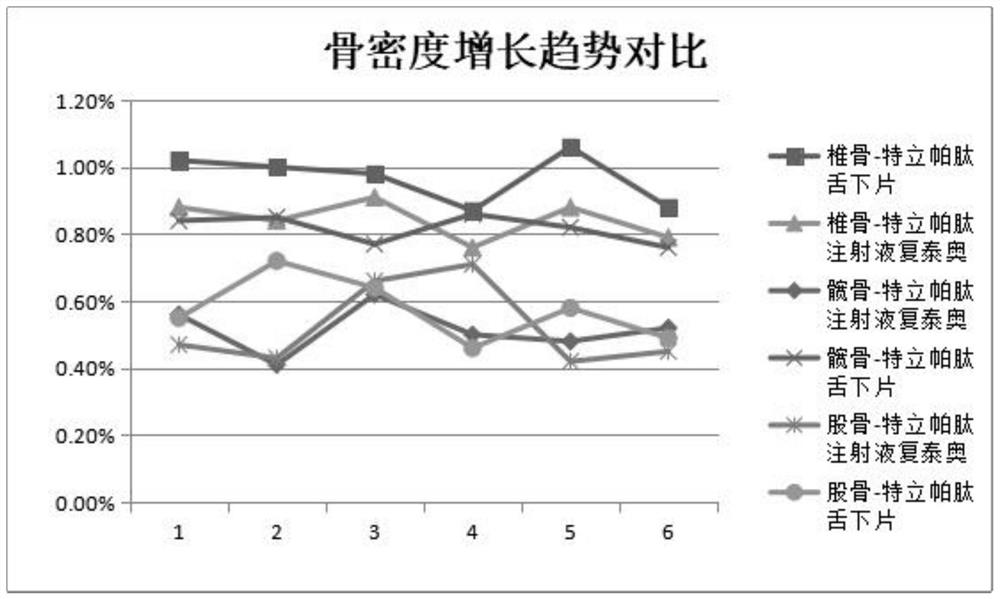
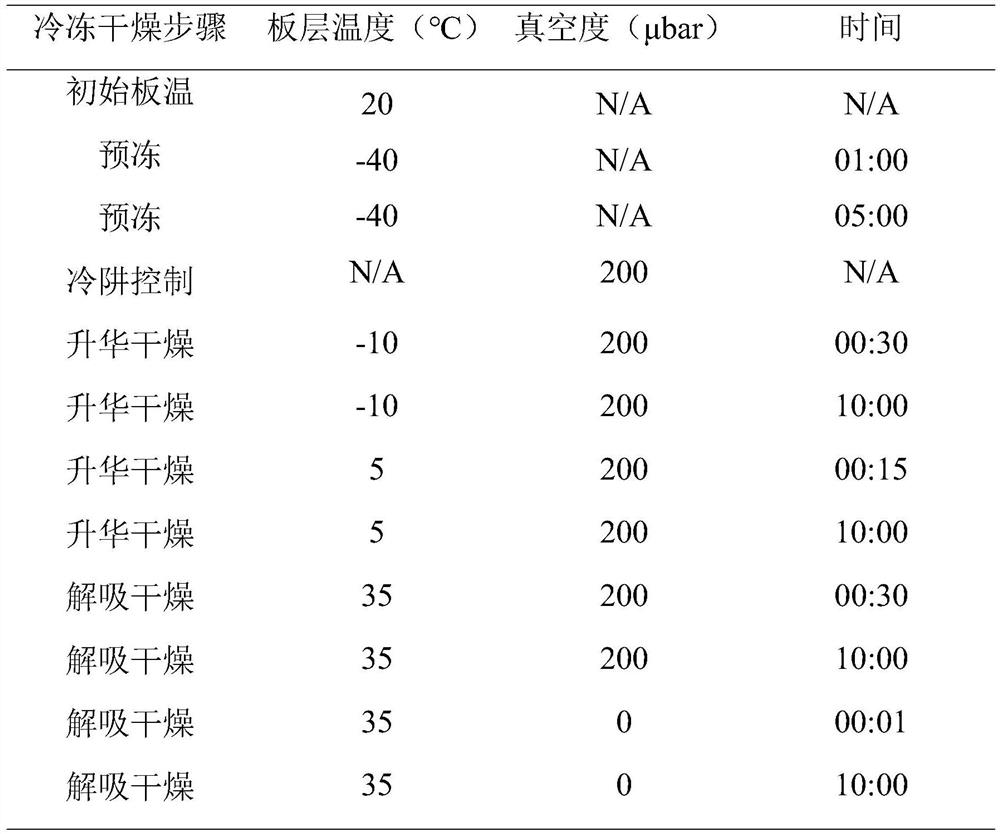
Tteriparatide freeze-dried pharmaceutical composition and preparation method thereof
A technology of teriparatide and composition, which is applied in the field of teriparatide freeze-dried pharmaceutical composition and preparation thereof, can solve the problem of high difficulty in using pre-filled injection pen, unsuitable preparation into sustained-release preparation, poor patient compliance, etc. to reduce the risk of sterility, reduce the risk of bacterial infection, and improve compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation: Weigh an appropriate amount of water to dissolve 5 mg of lactose and 50 mg of mannitol, dissolve the lactose and mannitol fully and uniformly, and mix to obtain a lactose-mannitol solution; weigh an appropriate amount of water to dissolve teriparatide raw materials, with a concentration of 2 mg / ml, after dissolving, add to lactose-mannitol solution, stir evenly to obtain lactose-mannitol-teriparatide solution; take appropriate amount of water to dissolve sodium acetate and glacial acetic acid, add lactose-mannitol-teriparatide after mixing In the solution, stir evenly, adjust the pH value to 4.0 with 10% sodium hydroxide or 10% hydrochloric acid according to the measured pH value, and continue stirring evenly. Weigh directly 0.5 mg of menthol and add it to the above solution, stir until it dissolves completely to obtain a composition solution, use a mould, pour the obtained composition solution into the mould, put it into a freeze dryer for lyophilization, ...
Embodiment 2
[0045] Preparation: Weigh an appropriate amount of water to dissolve 50 mg of lactose and 50 mg of mannitol, dissolve the lactose and mannitol fully and uniformly, and then mix to obtain a lactose-mannitol solution; weigh an appropriate amount of water to dissolve teriparatide raw materials, the concentration is 2 mg / ml, after dissolving, add to lactose-mannitol solution, stir evenly to obtain lactose-mannitol-teriparatide solution; take appropriate amount of water to dissolve sodium acetate and glacial acetic acid, add lactose-mannitol-teriparatide after mixing In the solution, stir evenly, adjust the pH value to 4.0 with 10% sodium hydroxide or 10% hydrochloric acid according to the measured pH value, and continue stirring evenly. Weigh directly 0.5 mg of menthol and add it to the above solution, stir until it dissolves completely to obtain a composition solution, use a mould, pour the obtained composition solution into the mould, put it into a freeze dryer for lyophilizatio...
Embodiment 3
[0047] Preparation: Weigh an appropriate amount of water to dissolve 10 mg of lactose and 100 mg of mannitol, dissolve the lactose and mannitol fully and uniformly, and then mix to obtain a lactose-mannitol solution; weigh an appropriate amount of water to dissolve teriparatide raw materials, with a concentration of 2 mg / ml, after dissolving, add to lactose-mannitol solution, stir evenly to obtain lactose-mannitol-teriparatide solution; take appropriate amount of water to dissolve sodium acetate and glacial acetic acid, add lactose-mannitol-teriparatide after mixing In the solution, stir evenly, adjust the pH value to 4.0 with 10% sodium hydroxide or 10% hydrochloric acid according to the measured pH value, and continue stirring evenly. Weigh directly 0.5 mg of menthol and add it to the above solution, stir until it dissolves completely to obtain a composition solution, use a mould, pour the obtained composition solution into the mould, put it into a freeze dryer for lyophiliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com