Process for preparation of anti-tubercular combination and pharmaceutical composition prepared therefrom
A pharmaceutical composition, tuberculosis technology, applied in the direction of antibacterial drugs, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of long disintegration time of tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0102] Example 1: The preparation method of the FDC composition of two layers of four kinds of drugs
[0103] These drugs: rifampicin, isoniazid, pyrazinamide, and ethambutol are available to those skilled in the art. For example, rifampicin, isoniazid, pyrazinamide, and ethambutol hydrochloride are available from BTX Global Pharmaceuticals, Inc. (New Jersey, US), F. Hoffmann-La Roche Ltd (New Jersey, US), respectively. ); Pharmchem Co. (New Delhi, India) and CBC (America) Corp (New York, US). The ingredients and their specific levels are listed in the table below.
[0104] Table 1: Formulations of RIP081231T
[0105] project
Element
% weight / weight
Tier I
1
rifampicin
14.02%
2
0.93%
3
0.93%
4
Granules - placebo
7.95%
Subtotal
23.83%
Tier II
5
polyethylene glycol
0.94%
6
Particl...
example 2
[0129] Example 2: Stability test of compositions of the present invention
[0130] Stability testing was performed by using the RIP080813T lozenge prepared in Example 1 under the conditions described in the guidelines of the International Conference on Harmonization. The conditions for the long-term test are 25°C and 60%RH. The conditions of the accelerated test are 40° C. and 75% RH. The results are shown in Table 1 below.
[0131] Table 1: Conditions for accelerated testing
[0132]
[0133]
[0134] As shown in the table, the FDC compositions of the present invention exhibit excellent stability.
example 3
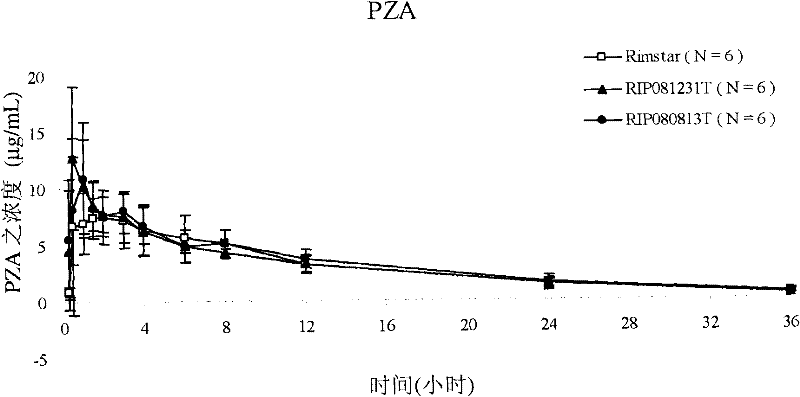
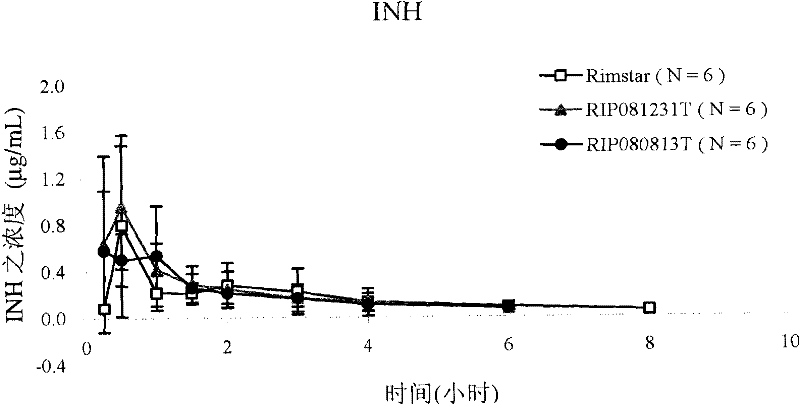
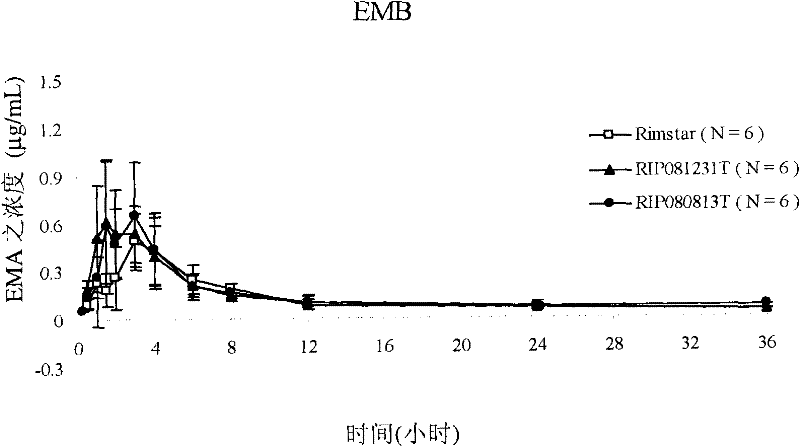
[0135] Example 3: Pharmacokinetic studies in humans in vivo
[0136] Conduct clinical trials. The individual is divided into three groups and tested respectively RIP080813T, RIP081231T (composition of the present invention among the example 1) and
[0137] Before administration, 10 ml of blood was collected from each individual as a blank control. In the trial, individuals were given two RIP080813T lozenges, one RIP081231T lozenge, and one RIP081231T lozenge at different times Lozenges and 10 ml of blood were collected from each subject. Rifampicin, isotropic acid and isotropic acid were calculated from blood concentrations obtained at time intervals after administration (i.e., at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 and 36 hours). Pharmacokinetic parameters (Cmax and AUC) of nicotinic acid hydrazine, pyrazinamide and ethambutol hydrochloride.
[0138] The results shown in Table 3 below demonstrate that compared to RIP080813T and RIP081231T of the present invention ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com