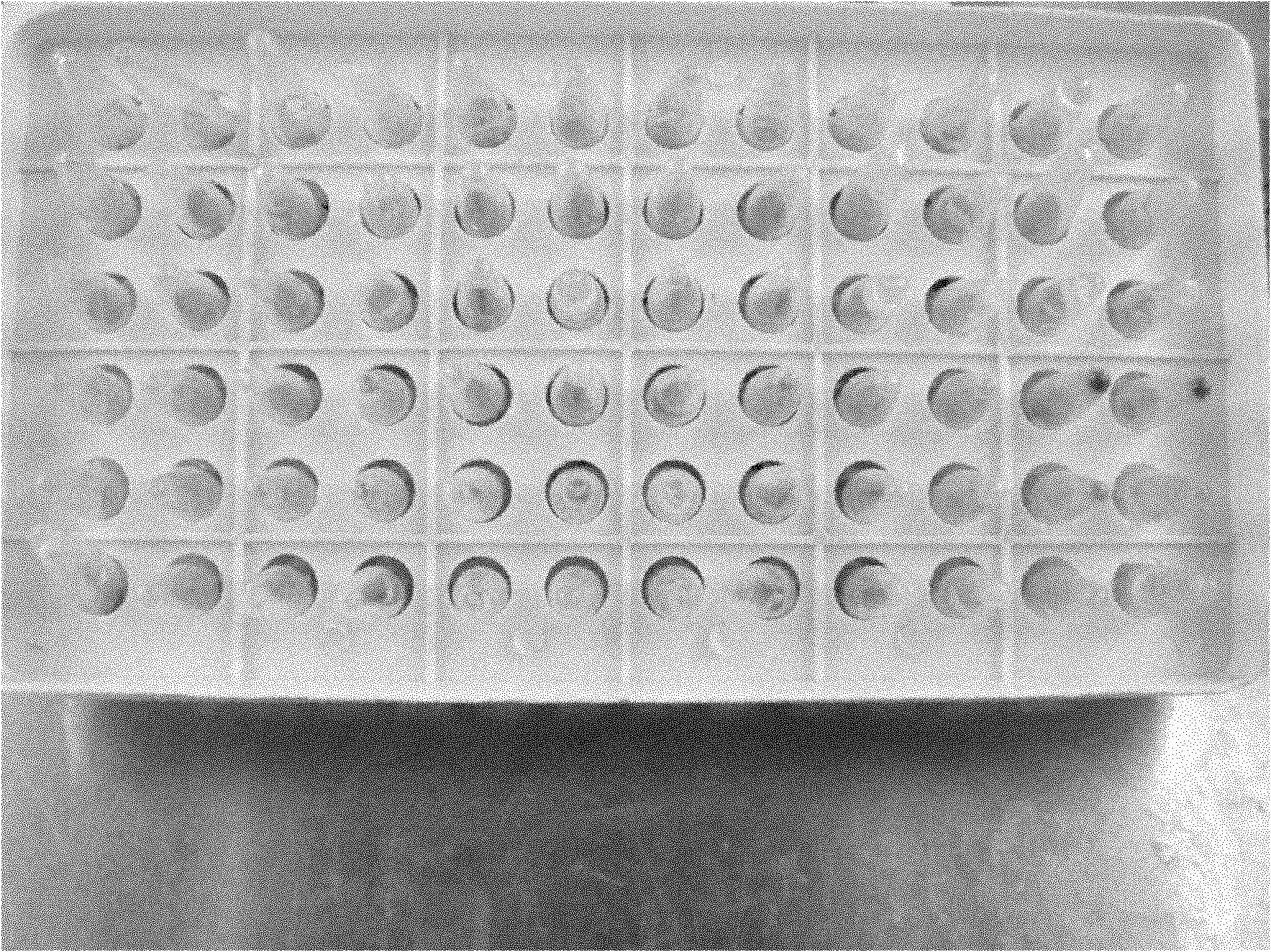Patents
Literature
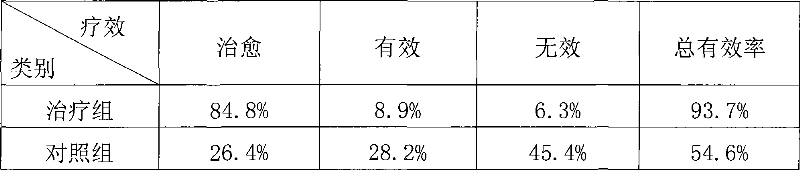
426 results about "Anti tubercular" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antitubercular [-too͡bur′kyələr] any agent or group of drugs used to treat tuberculosis. At least two drugs, and usually three, are required in various combinations in pulmonary tuberculosis therapy.
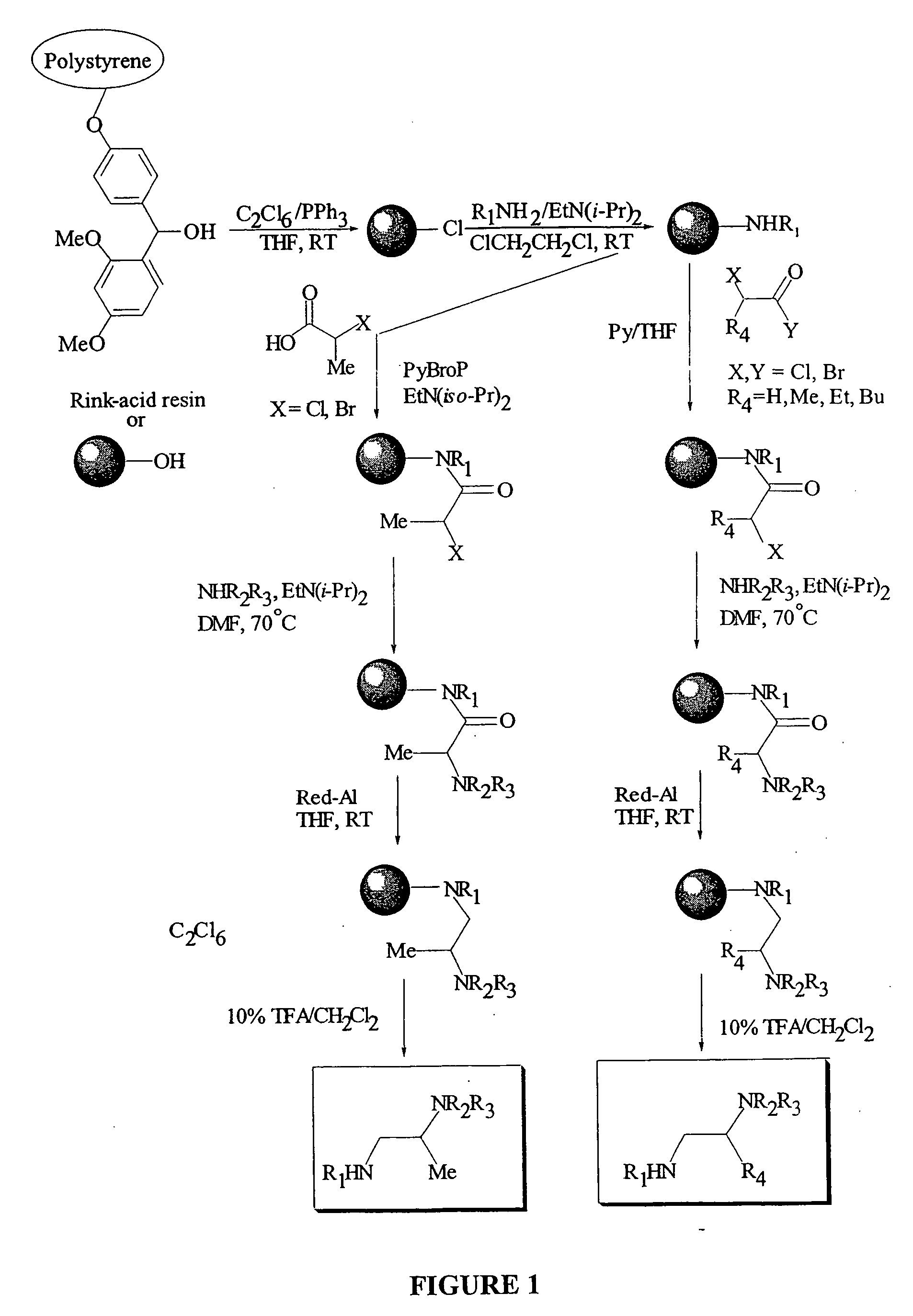
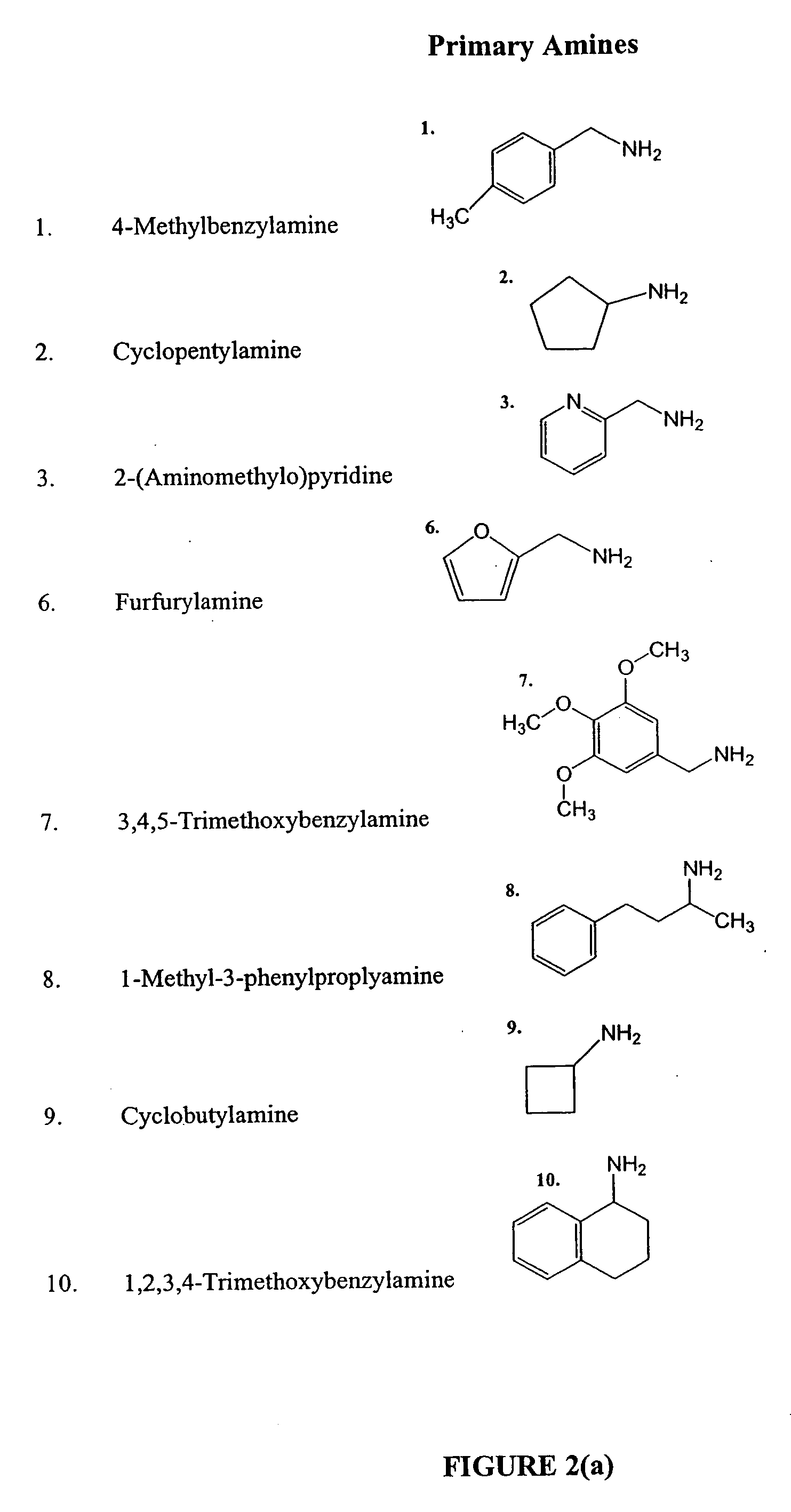
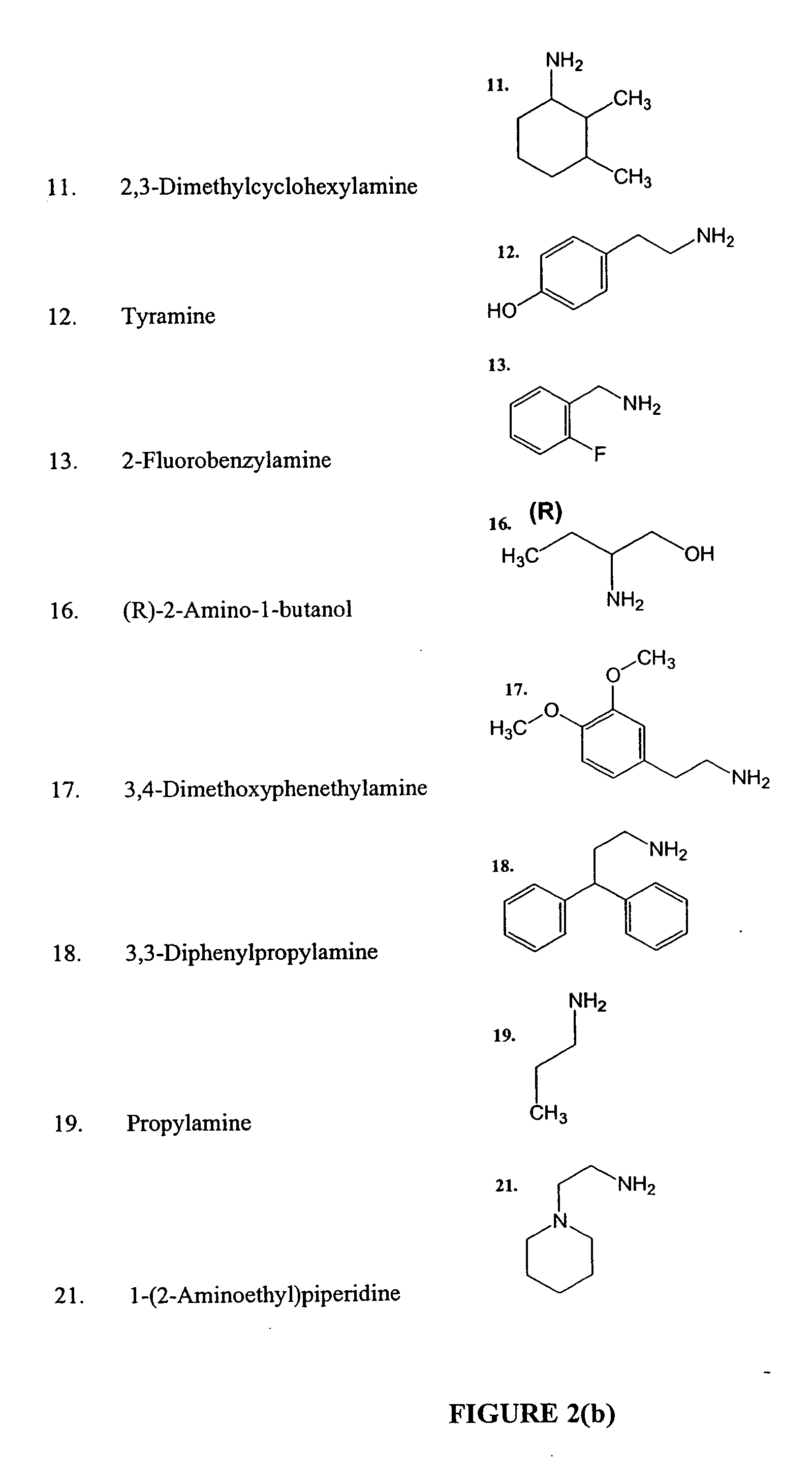
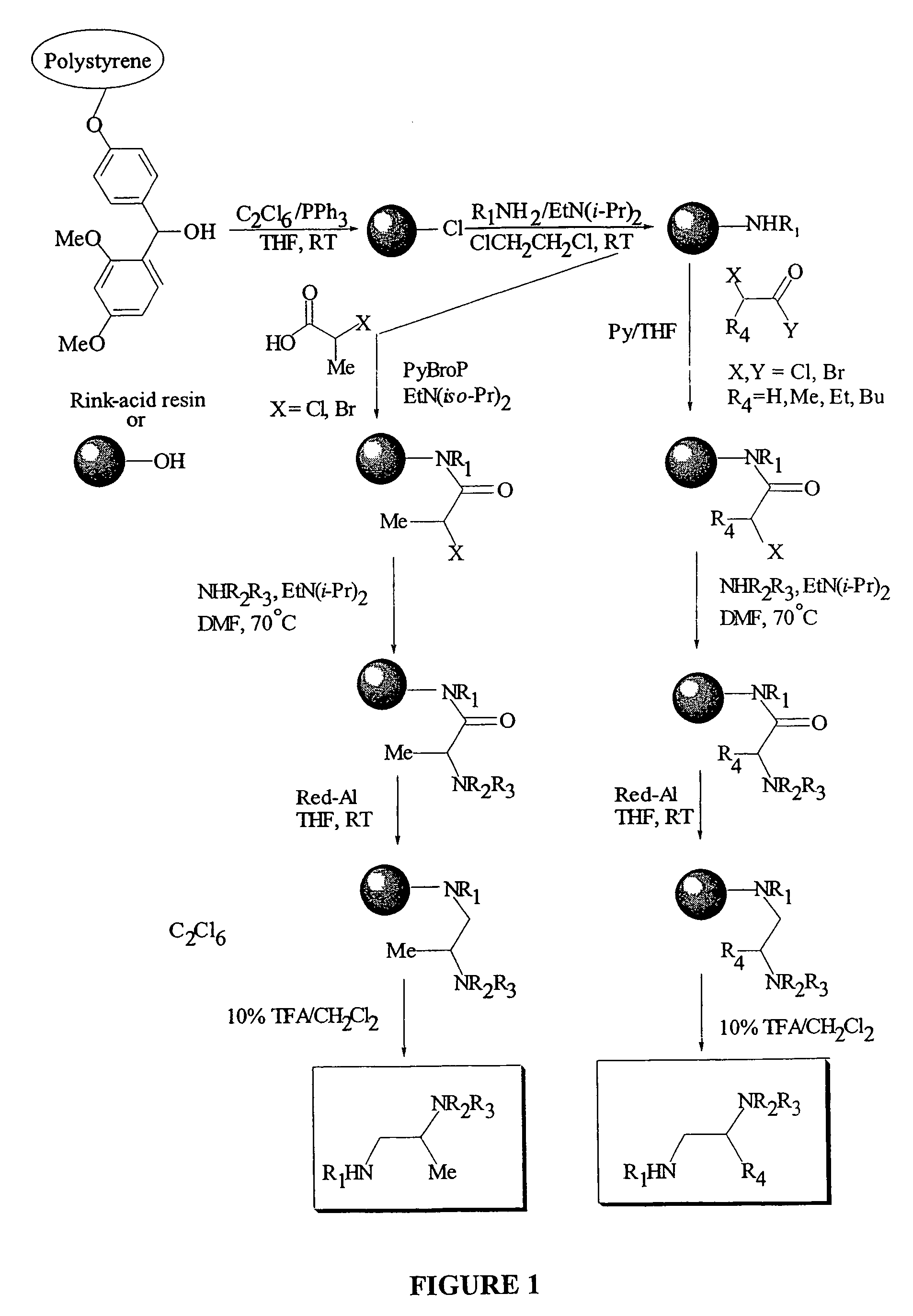
Anti tubercular drug: compositions and methods
Methods and compositions for treating disease caused by infectious agents, particularly tuberculosis. In particular, methods and compositions comprising substituted ethylene diamines for the treatment of infectious diseases are provided. In one embodiment, these methods and compositions are used for the treatment of mycobacterial infections, including, but not limited to, tuberculosis.
Owner:SEQUELLA +1
Fluoroquinolone acetal isoniazone, and preparation method and application thereof
InactiveCN102827187ASmall side effectsReduce the chance of developing drug resistanceAntibacterial agentsOrganic active ingredientsOxygen atomSide effect
The invention discloses a fluoroquinolone acetal isoniazone, of which the chemical structure general formula is disclosed as Formula I shown in the description, wherein R1 is hydrogen atom or methyl group; R2 is hydrogen atom or amino group; R3 is hydrogen atom, methyl group, ethyl group, formacyl group, acetyl group, aroyl group or sulfonyl group; R4 is hydrogen atom or methyl group; and X is oxygen atom or sulfur atom. The fluoroquinolone isoniazone disclosed by the invention implements complementarity between the two anti-tuberculosis medicines fluoroquinolone and the isoniazide, lowers the toxic and side effect of the fluoroquinolone and the isoniazide, reduces the generation probability of drug resistance of Mycobacterium tuberculosis for the double-effect antimicrobial agent, and can be used as an anti-Mycobacterium tuberculosis medicine for brand-new structure development of medicinal active substances.
Owner:HENAN UNIVERSITY
Compound for inhibiting mycobacterium tuberculosis, screening method and uses thereof
InactiveCN102732474AInhibitory activityReduce workloadAntibacterial agentsOrganic active ingredientsDiseaseTyrosine
The present invention discloses a screening method of a compound for inhibiting mycobacterium tuberculosis. The method comprises the following steps: (1) establishing a screening model adopting tyrosine phosphatase as a target point: adopting genome DNA of tubercle bacillus H37 Rv as a template, adopting a PCR technology to clone tyrosine phosphatase gene, transforming host cells, culturing the transformant to obtain recombinant tyrosine phosphatase from the culture, carrying out enzyme activity analysis, and establishing the screening model adopting tyrosine phosphatase as the target point; and (2) adopting the screening model established in the step (1) to screen a compound providing inhibition activity for the tyrosine phosphatase. With the method of the present invention, the tyrosine phosphatase inhibitor with anti-mycobacterium tuberculosis effect can be rapidly and efficiently screened. In addition, the present invention further discloses a compound screened by using the screening method, and uses of the compound in preparations of drugs for treatments of diseases, wherein the inducement or one of the inducements of the diseases is the tyrosine phosphatase.
Owner:SUN YAT SEN UNIV
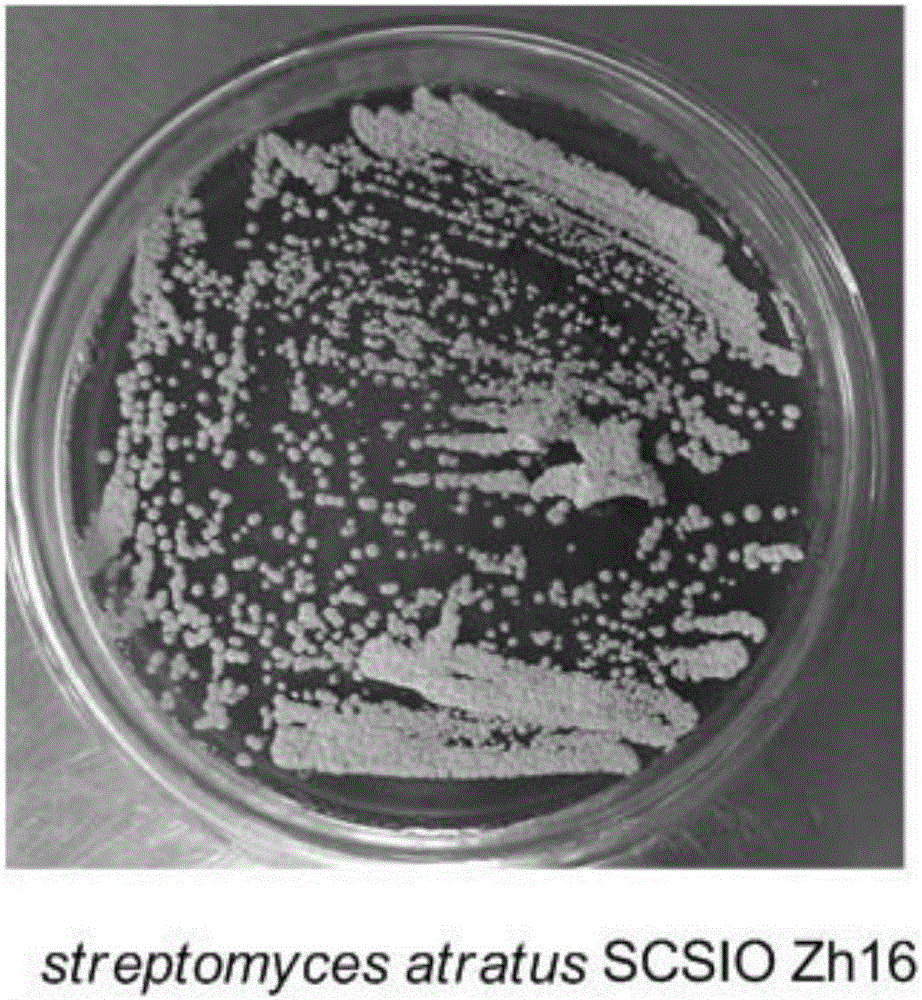
Streptomyces atratus and application of cyclic peptide compounds with same to preparing mycobacterium tuberculosis resistant medicines
ActiveCN106279370AGood inhibitory effectAntibacterial agentsBacteriaCyclic peptideAntituberculous drugs
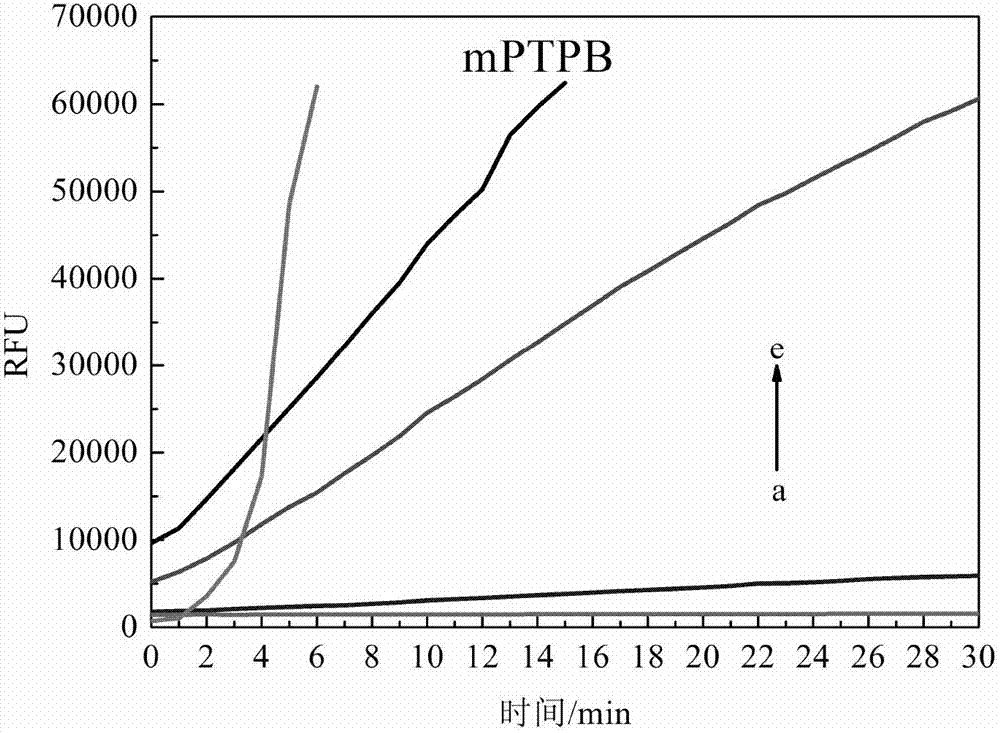
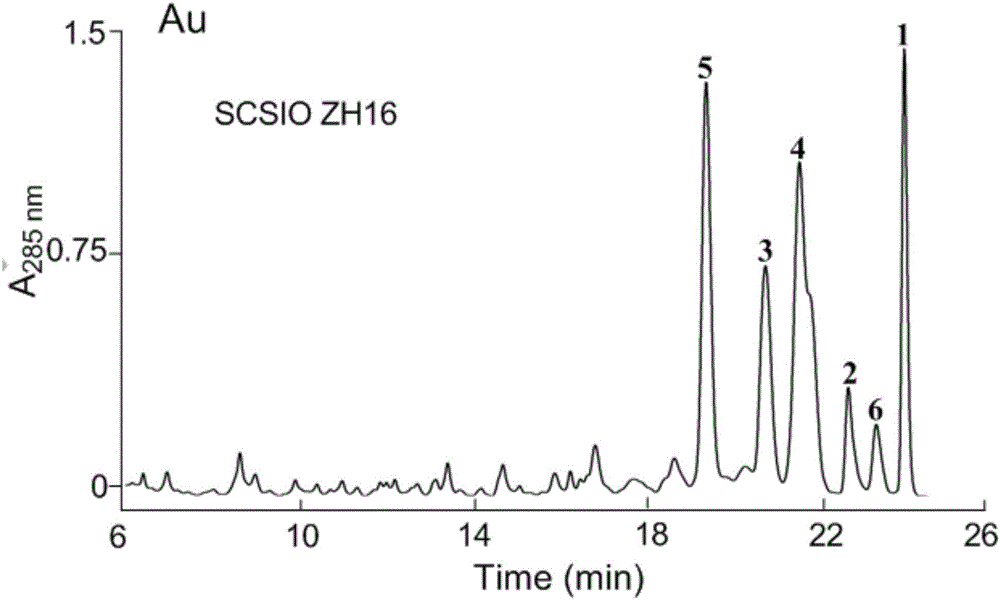
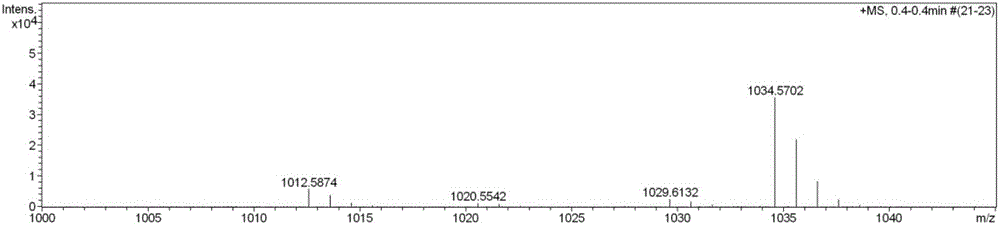
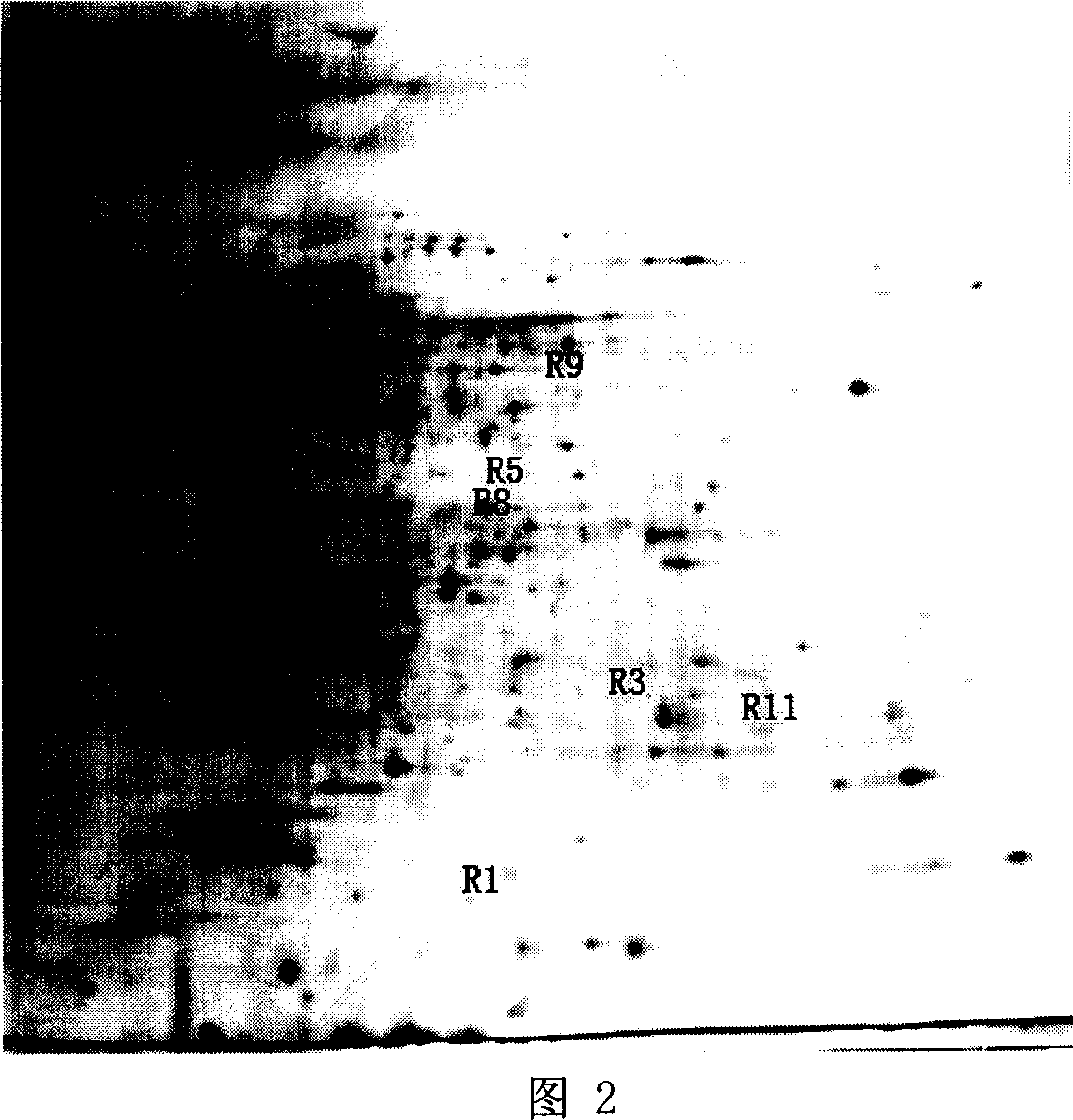
The invention discloses streptomyces atratus and application of cyclic peptide compounds with the same to preparing mycobacterium tuberculosis resistant medicines. A structural formula of the cyclic peptide compounds is shown. A preservation number of the streptomyces atratus SCSIO Zh16 is CGMCC No.12198. The streptomyces atratus and the application have the advantages that the six cyclic peptide compounds are obtained from fermentation cultivation substances of the streptomyces atratus SCSIO Zh16 by means of separation, the cyclic peptide compound 6 is high in mycobacterium tuberculosis resistant activity, obvious effects of inhibiting mycobacterium tuberculosis can be realized by the cyclic peptide compound, accordingly, the cyclic peptide compounds can be used for preparing anti-tuberculosis medicines and can be used for treating tuberculosis, alternative compounds can be provided for developing novel anti-tuberculosis medicines, and the streptomyces atratus and the application have important significance on developing marine medicinal materials in China.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI +1
Anti tubercular drug: compositions and methods
Methods and compositions for treating disease caused by infectious agents, particularly tuberculosis. In particular, methods and compositions comprising substituted ethylene diamines for the treatment of infectious diseases are provided. In one embodiment, these methods and compositions are used for the treatment of mycobacterial infections, including, but not limited to, tuberculosis.
Owner:SEQUELLA INC +1
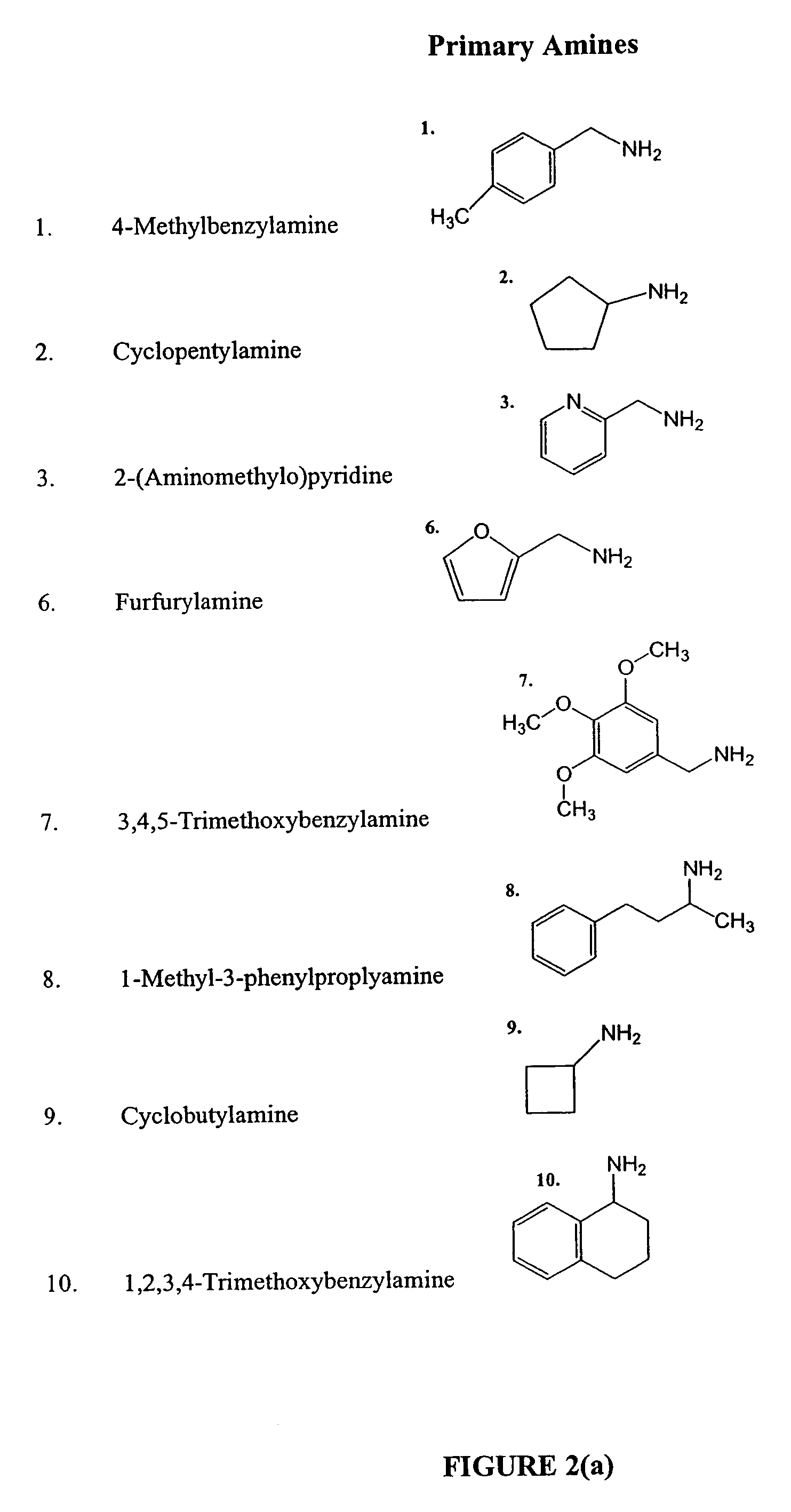
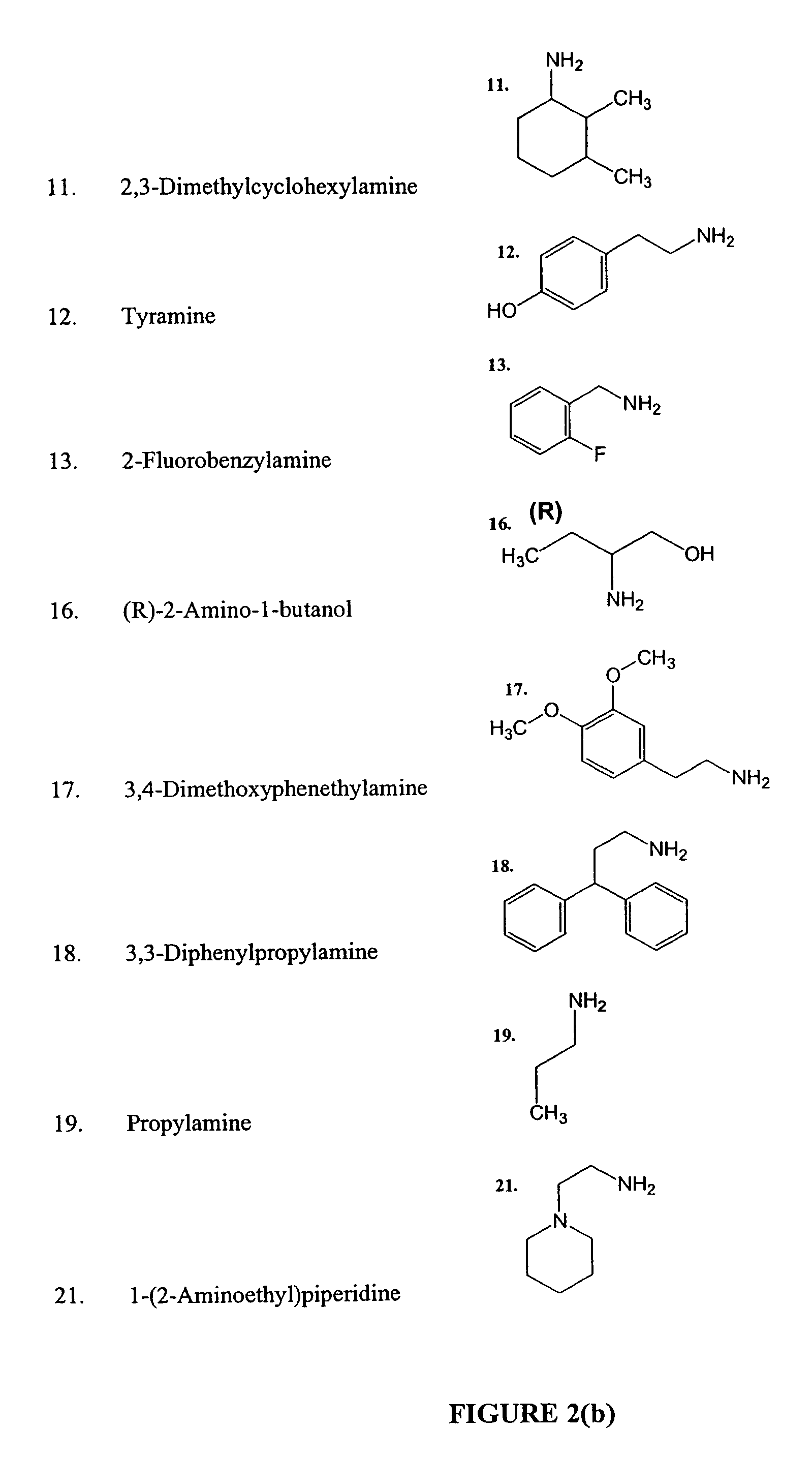
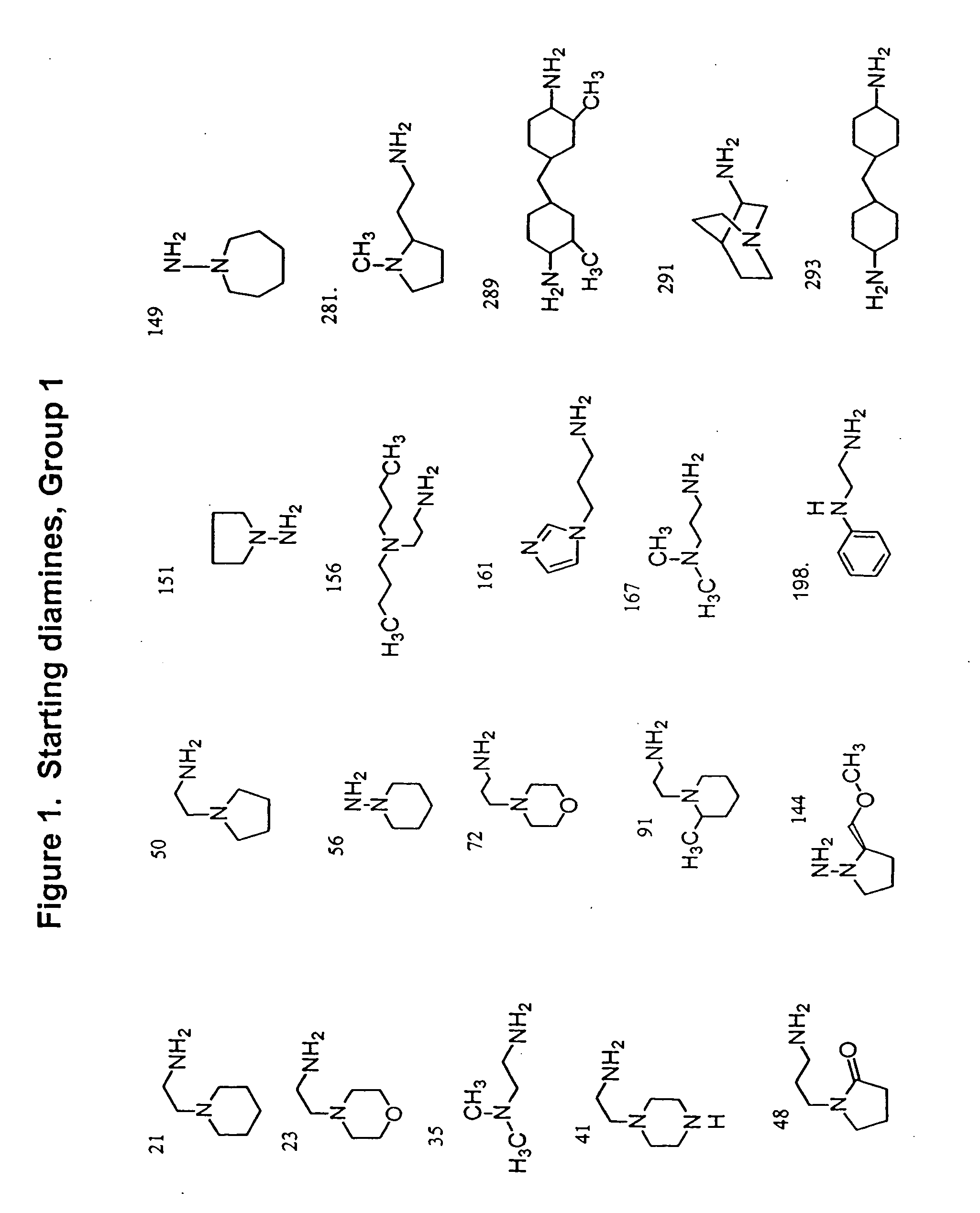
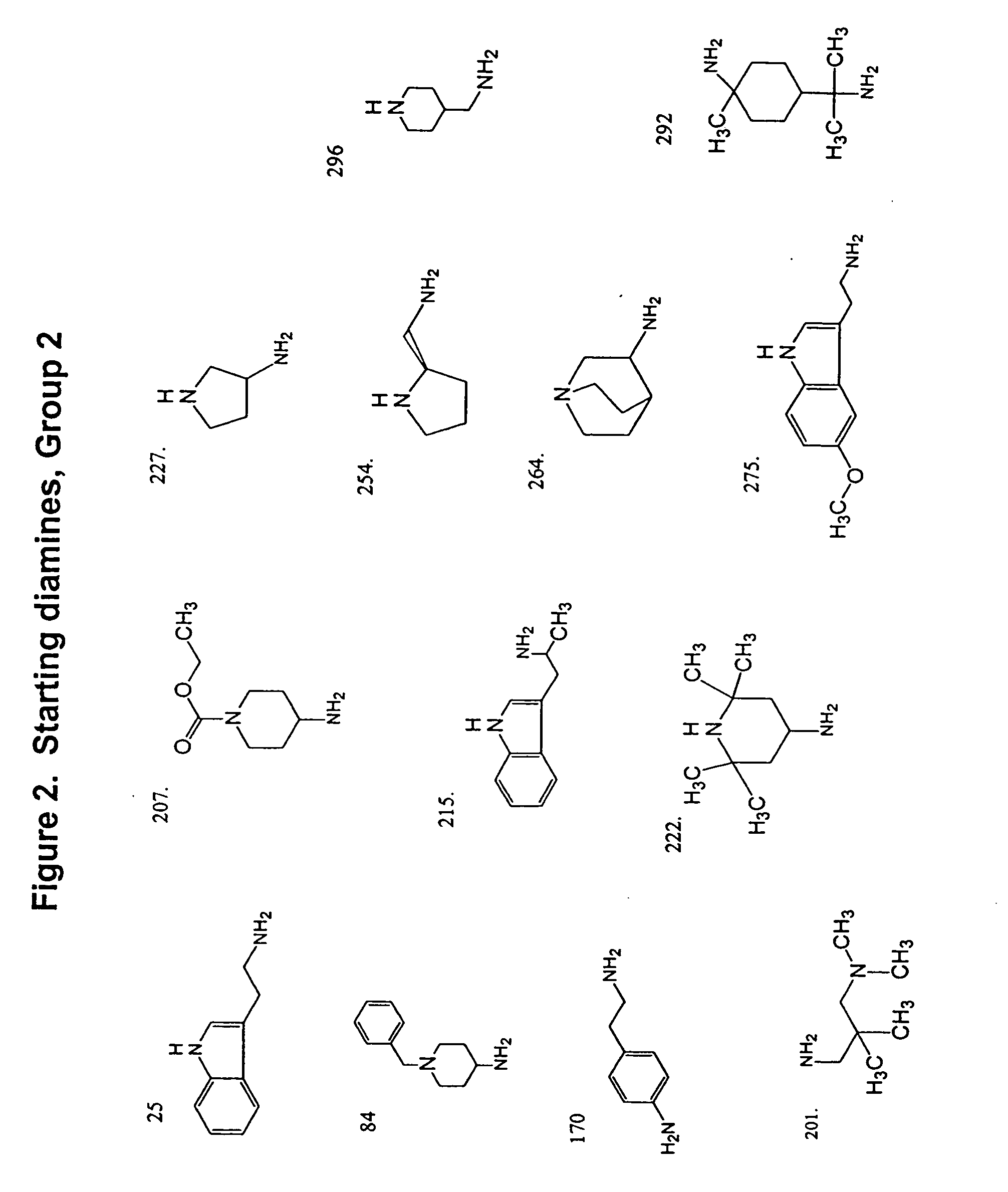
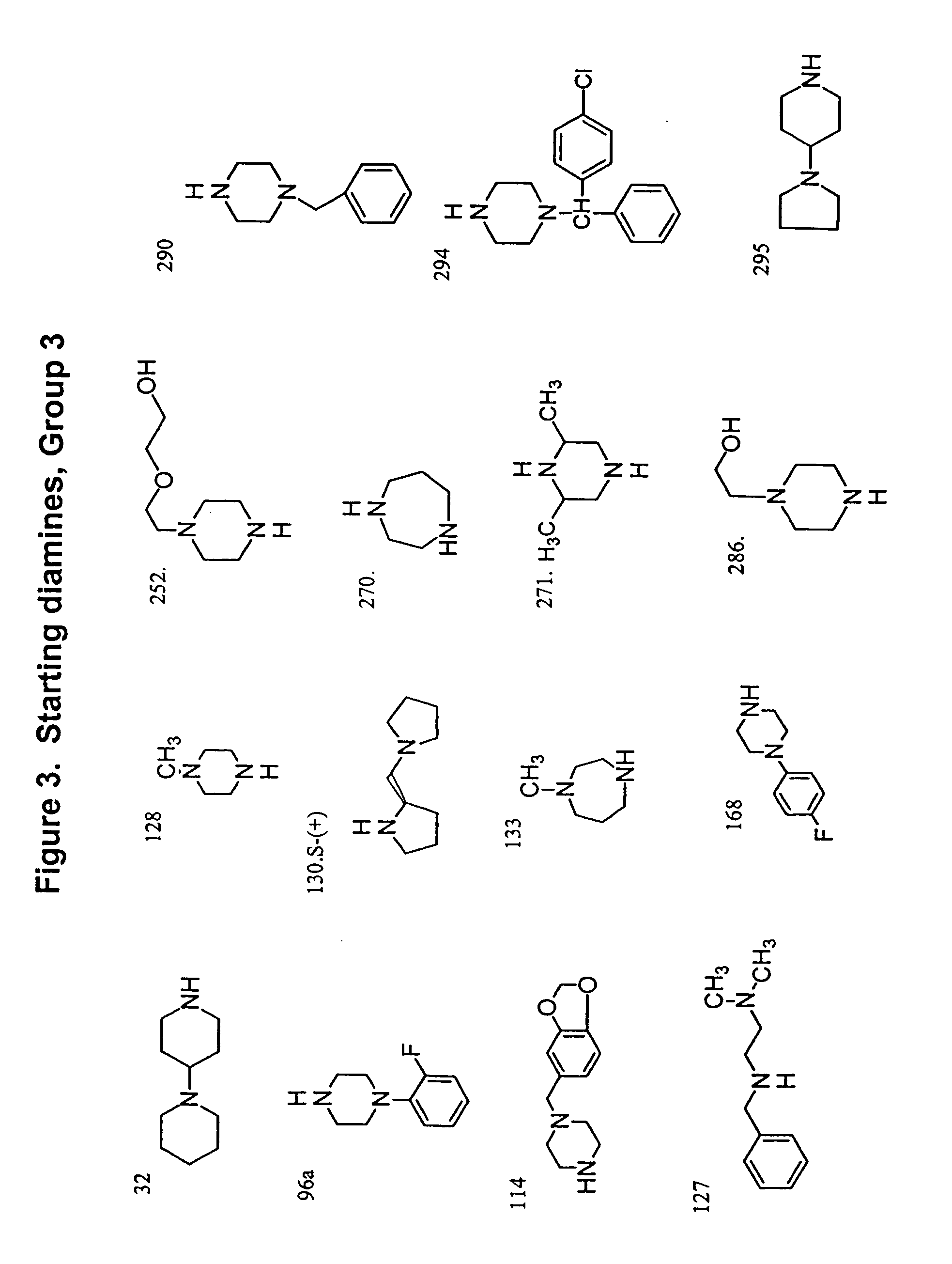
Methods and compositions comprising diamines as new anti-tubercular therapeutics
InactiveUS20050113574A1Improved anti-mycobacterial activityImproved anti-tuberculosis activityBiocideOrganic compound preparationMycobacterium InfectionsInfectious agent
Methods and compositions for treating disease caused by infectious agents, particularly tuberculosis. In particular, methods and compositions comprising novel diamine compositions for the treatment of infectious diseases are provided. In one embodiment, these methods and compositions are used for the treatment of mycobacterial infections, including, but not limited to, tuberculosis.
Owner:SEQUELLA
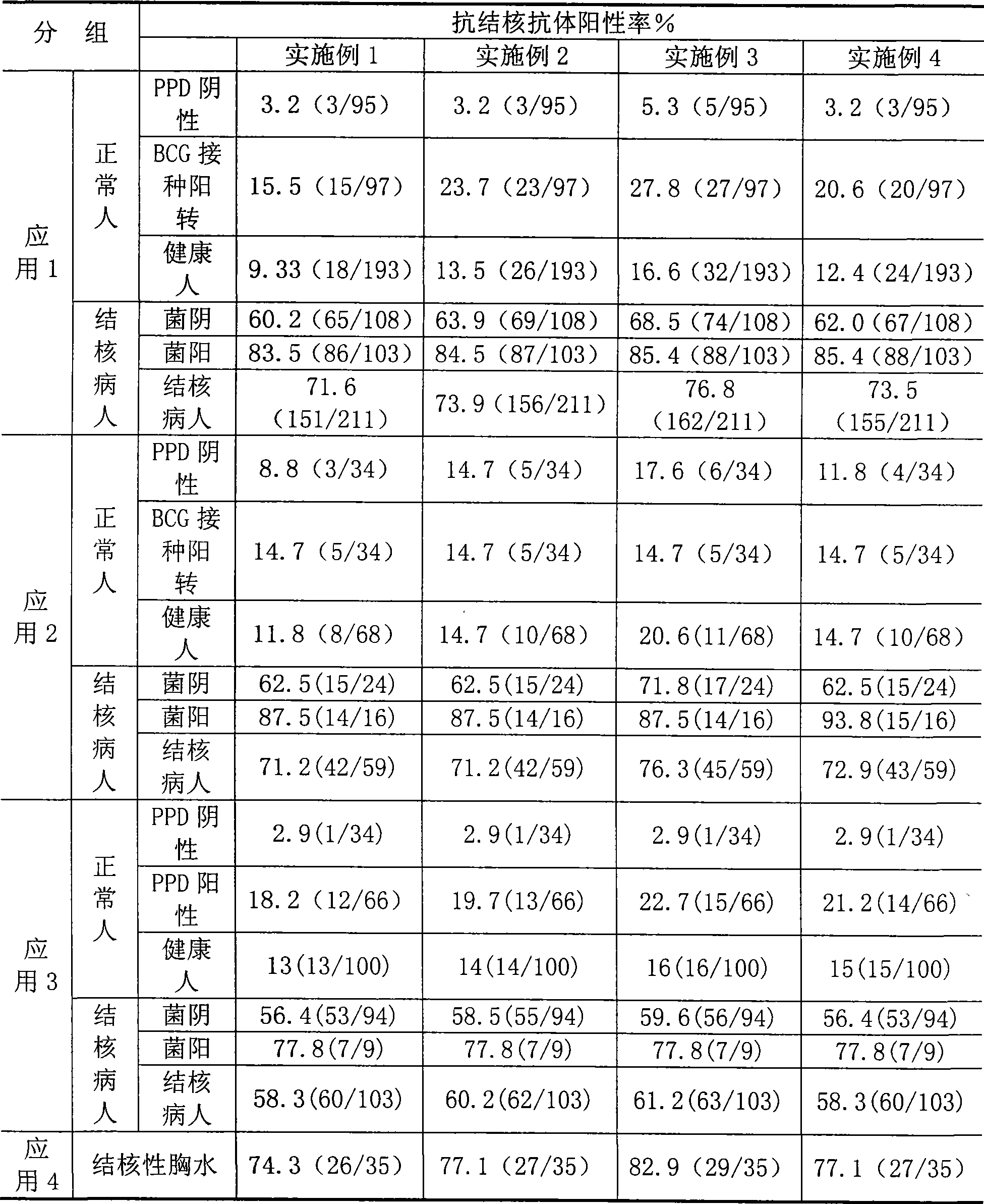
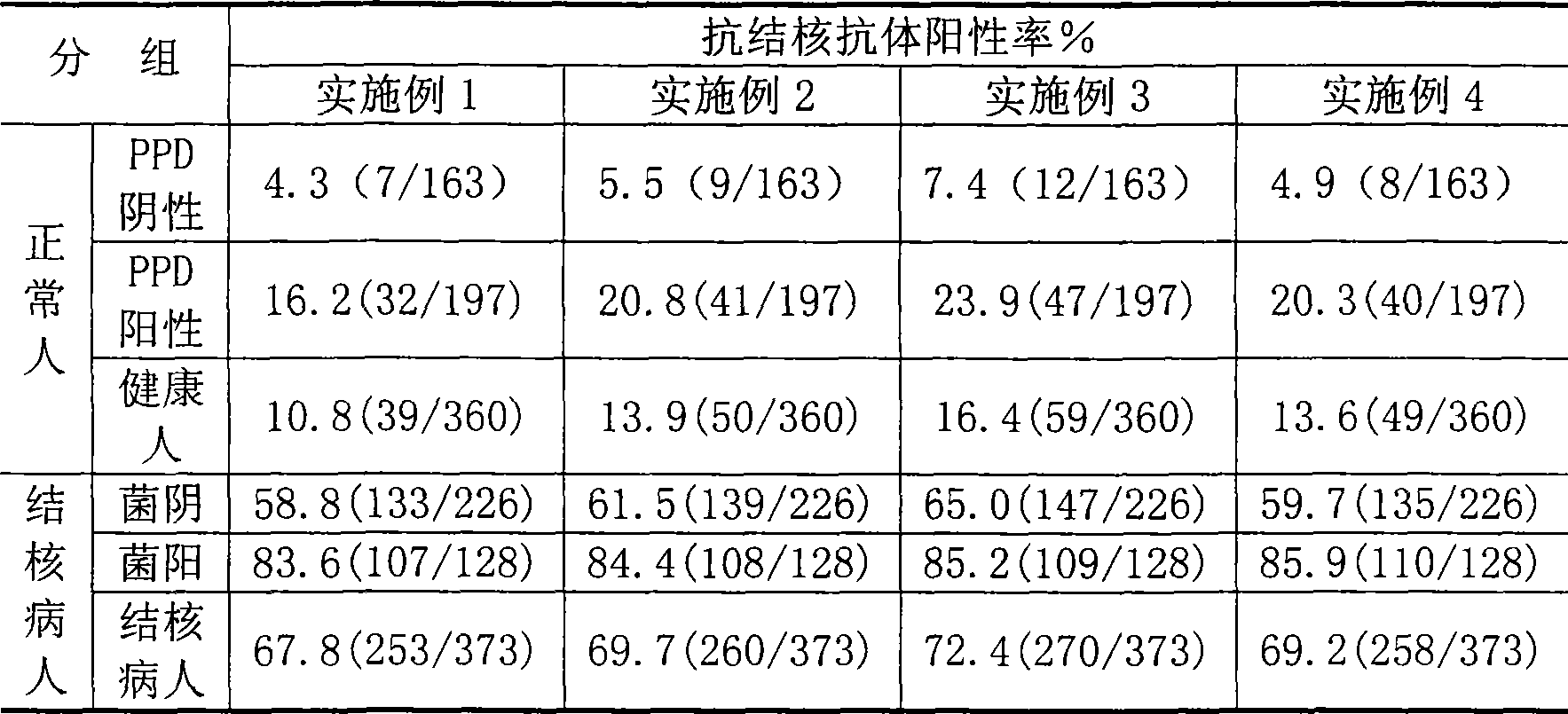
Tuberculosis antibody multi-antigen ELISA detecting kit and making method
The invention relates to a tuberculosis antibody multiple antigen ELISA detection kit and a preparation method thereof, which pertains to the field of tuberculosis medical immunology diagnostic techniques and mainly uses detection antigen, enzyme-linked antihuman IgG antibodies, substrates, positive control serum of tuberculosis patients, control serum of normal person, calf serum and polystyrene microplates to form the kit, wherein, the detection antigen adopts the mycobacterium tuberculosis complex strains of lipid Arabian mannose (LAM), 38kD and 16kD to be combined with arbitrary one or more than one mycobacterium tuberculosis recombinant proteins in the recombinant proteins of MPT63, MTB48 and CFP10-ESAT6. The mycobacterium tuberculosis has high sensitivity, strong specificity and complementarity, can be used for detecting specific antitubercular antibodies in such body fluid samples as serum, hydrothorax and the like, and assisting the diagnosis and differential diagnosis of tuberculosis.
Owner:中国人民解放军总医院第二附属医院
Tubercle bacillus recombined protein and expressing purefying method and application thereof
The invention relates to anti-tubercule bacillus recombination protein. It belongs to gene recombination technique field. Its manufacturing includes the following steps: combining antigen the 190-198 polypeptides of the Ag85B-Mpt64 with Mtb8.4; cloning into escherichia coli vector to express; further purifying to gain. It is recorded as Ag85B-Mpt64190-198-Mtb8.4(SEQ.ID.NO.1). The protein can be used as anti tubercule bacillus subunit vaccine.
Owner:FUDAN UNIV
Method for screening Mycobacterium tuberculosis drug-resistant protein
InactiveCN1945324AIncrease the burdenAccurate separation and identificationMicrobiological testing/measurementBiological testingVaccine antigenMicrobiology
The present invention belongs to the field of screening antituberculotic target, vaccine antigen and detecting target in molecular biological technology, and is especially process of screening protein resisting rifampicin as antituberculotic. The process includes the first culturing drug resistant strain, the subsequent separating protein of drug resistant strain from protein of sensitive strain, comparing protein of drug resistant strain and protein of sensitive strain to determine the drug resistant protein, and final separating and identifying drug resistant protein. The process of the present invention can separate and identify drug resistant protein of Mycobacterium tuberculosis to provide new way for further understanding the drug resisting mechanism of Mycobacterium tuberculosis, fast clinical detection of drug resistant strain, and developing vaccine and medicine.
Owner:FUDAN UNIV
Genetic engineering bacterial strain for directionally producing compounds having anti-tuberculosis activity and anti-tumor activity and application thereof
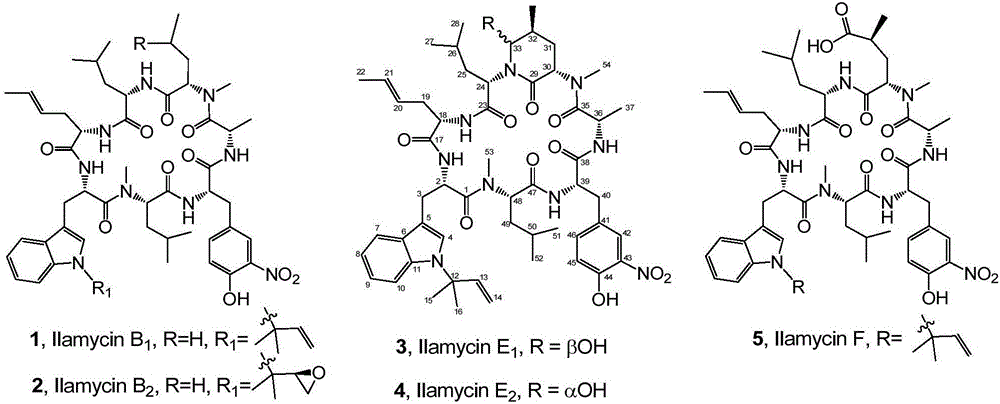
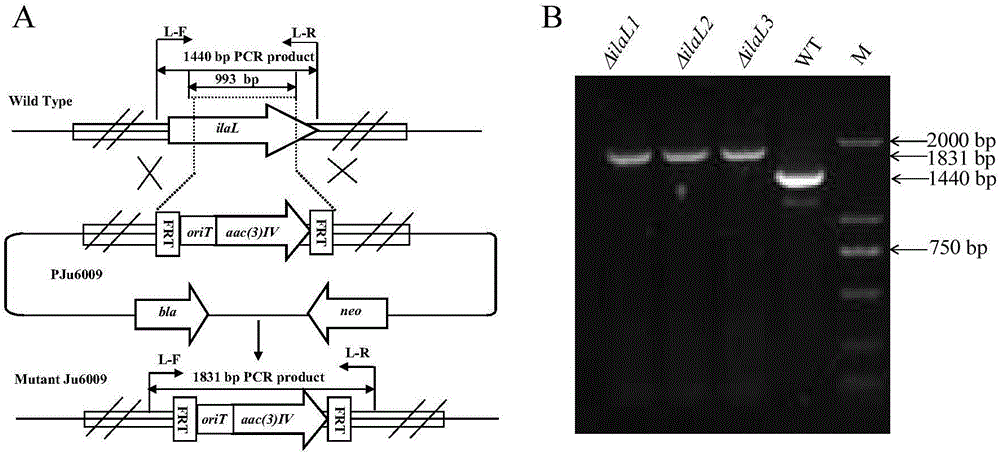
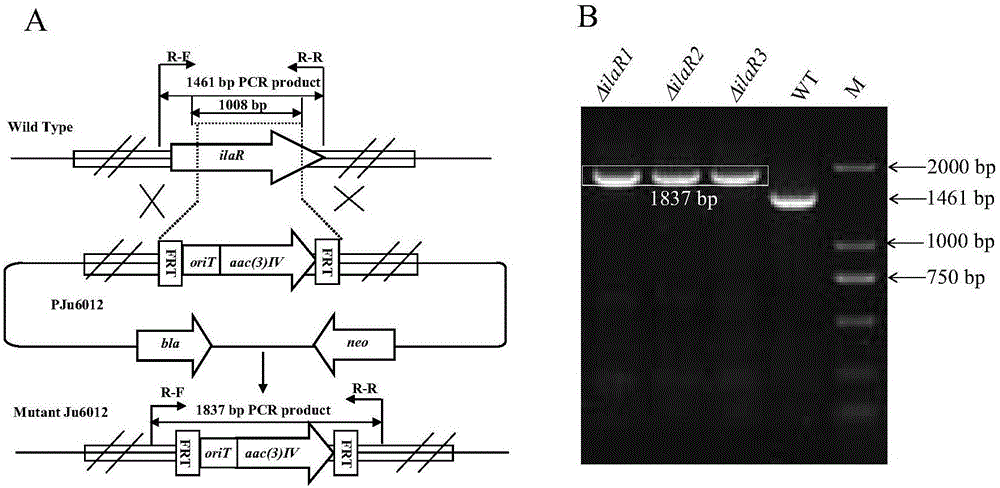
The invention discloses a genetic engineering bacterial strain for directionally producing compounds having anti-tuberculosis activity and anti-tumor activity and an application thereof. The genetic engineering bacterial strain is a genetic engineering bacterial strain obtained by knocking out an ialL gene or ilaR gene in a genome of streptomyces atratus SCSIO ZH16, wherein the ialL gene has the nucleotide sequence shown in SEQ ID NO.1, and the ialR gene has the nucleotide sequence shown in SEQ ID NO.2. The genetic engineering bacterial strain can produce the compounds 1, 2, 3, 4 and 5 having anti-tuberculosis activity and anti-tumor activity and shows great value in development of anti-tuberculosis drugs. Therefore, the successful construction of the genetic engineering bacterial strain for directionally producing the compounds having anti-tuberculosis activity and anti-tumor activity can accelerate the process of industrialization of the compounds and promote the development of Chinese marine drugs.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
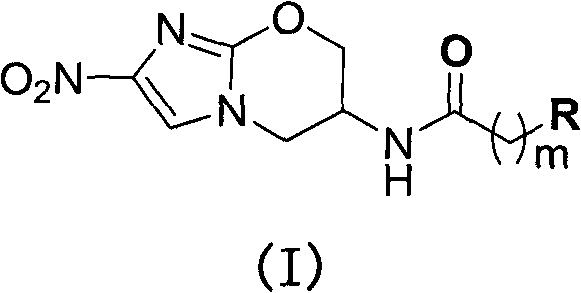
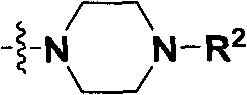
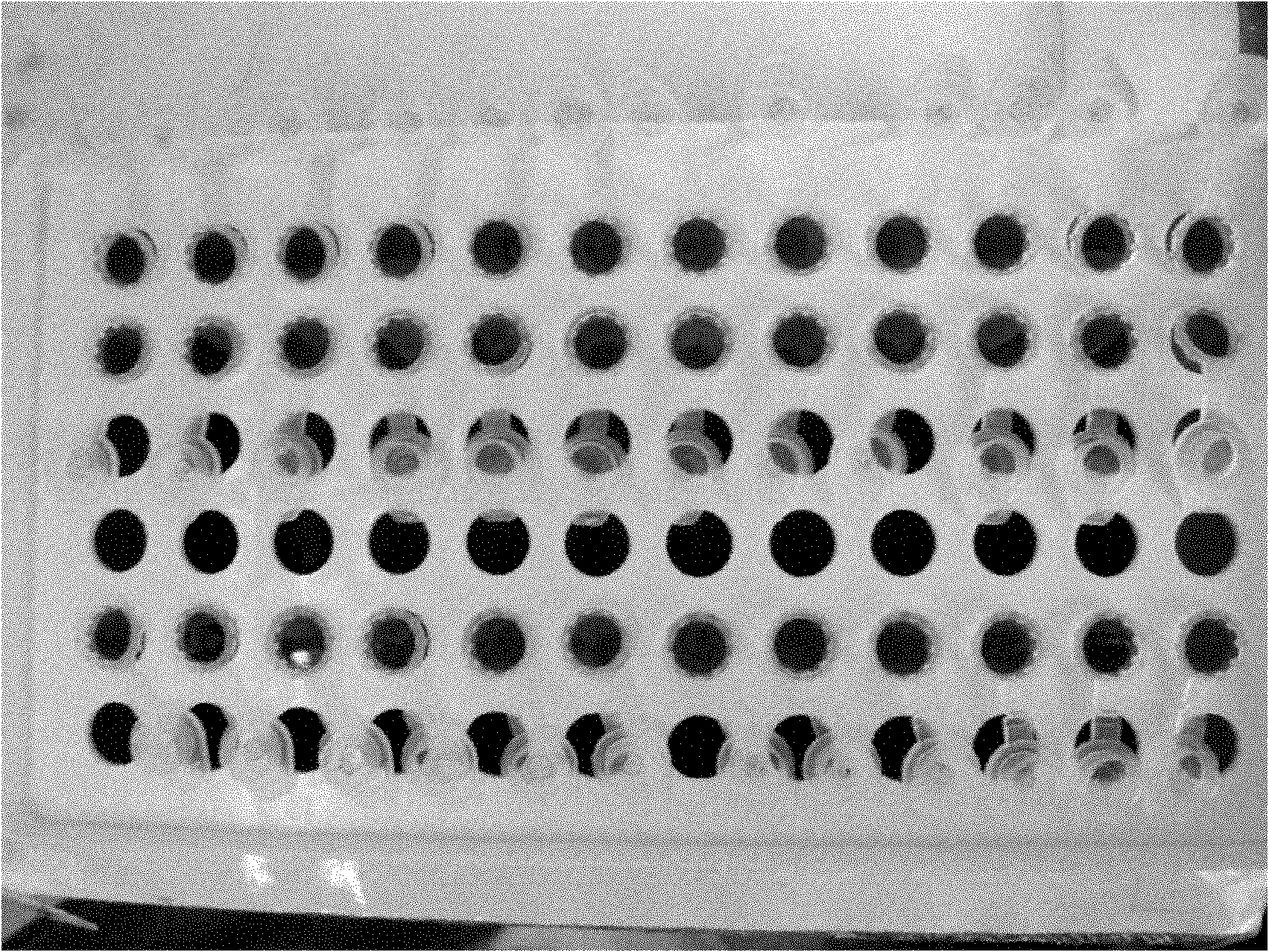
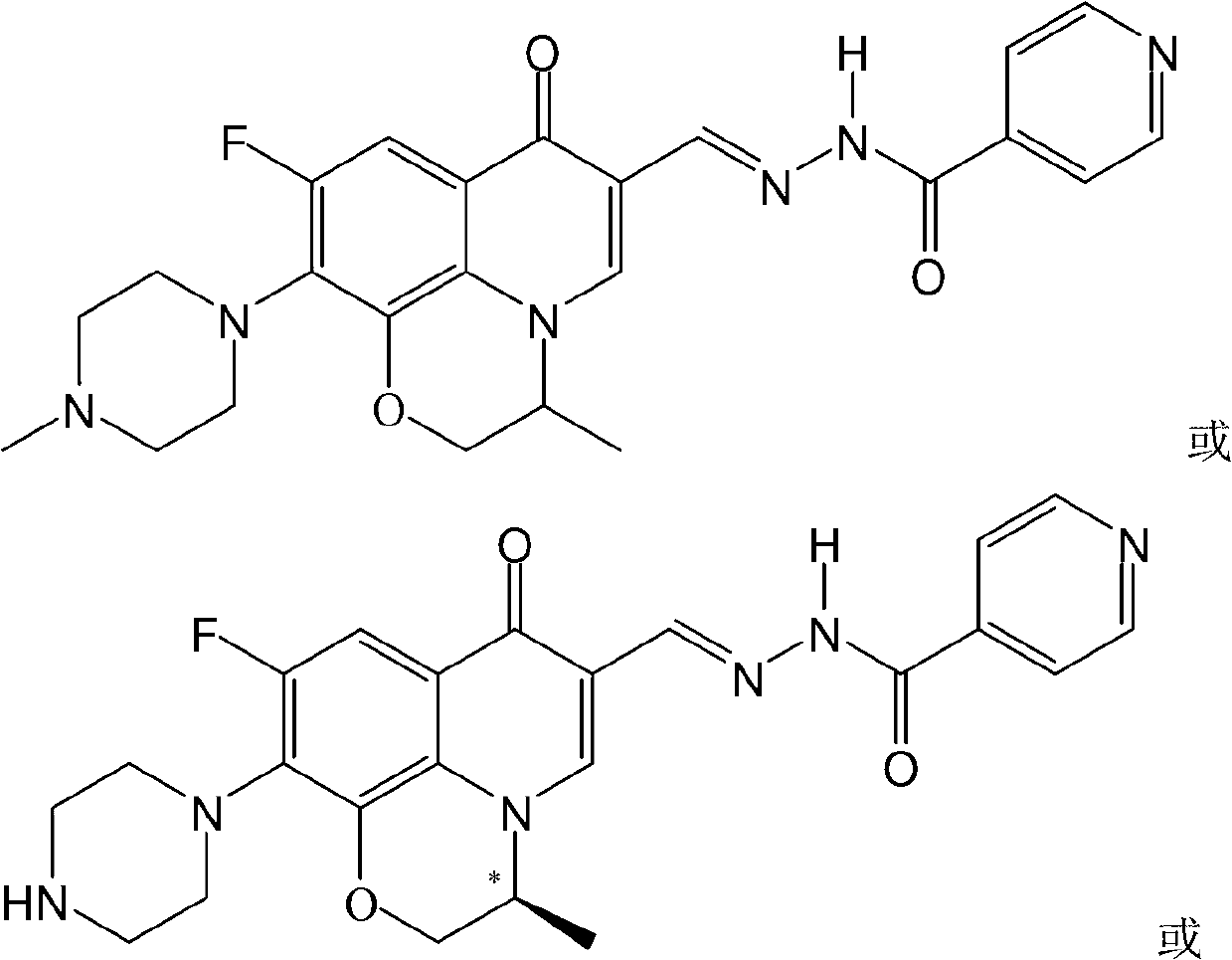
Pyrazolo[1, 5-a]pyridine compound and use thereof
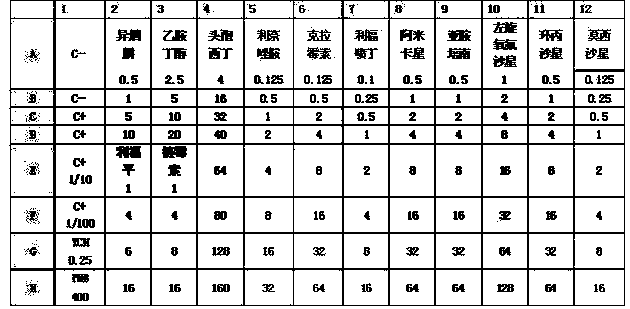
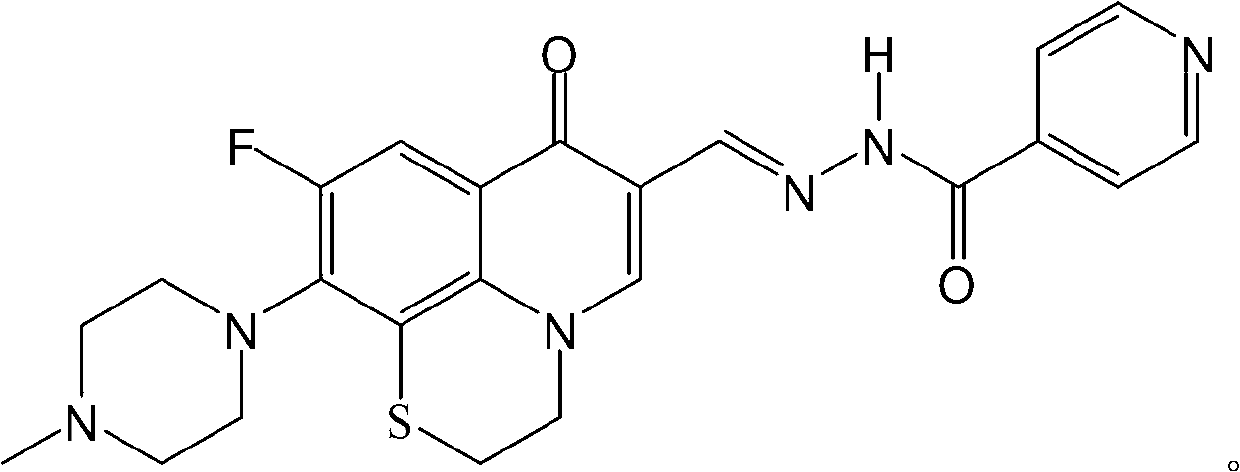
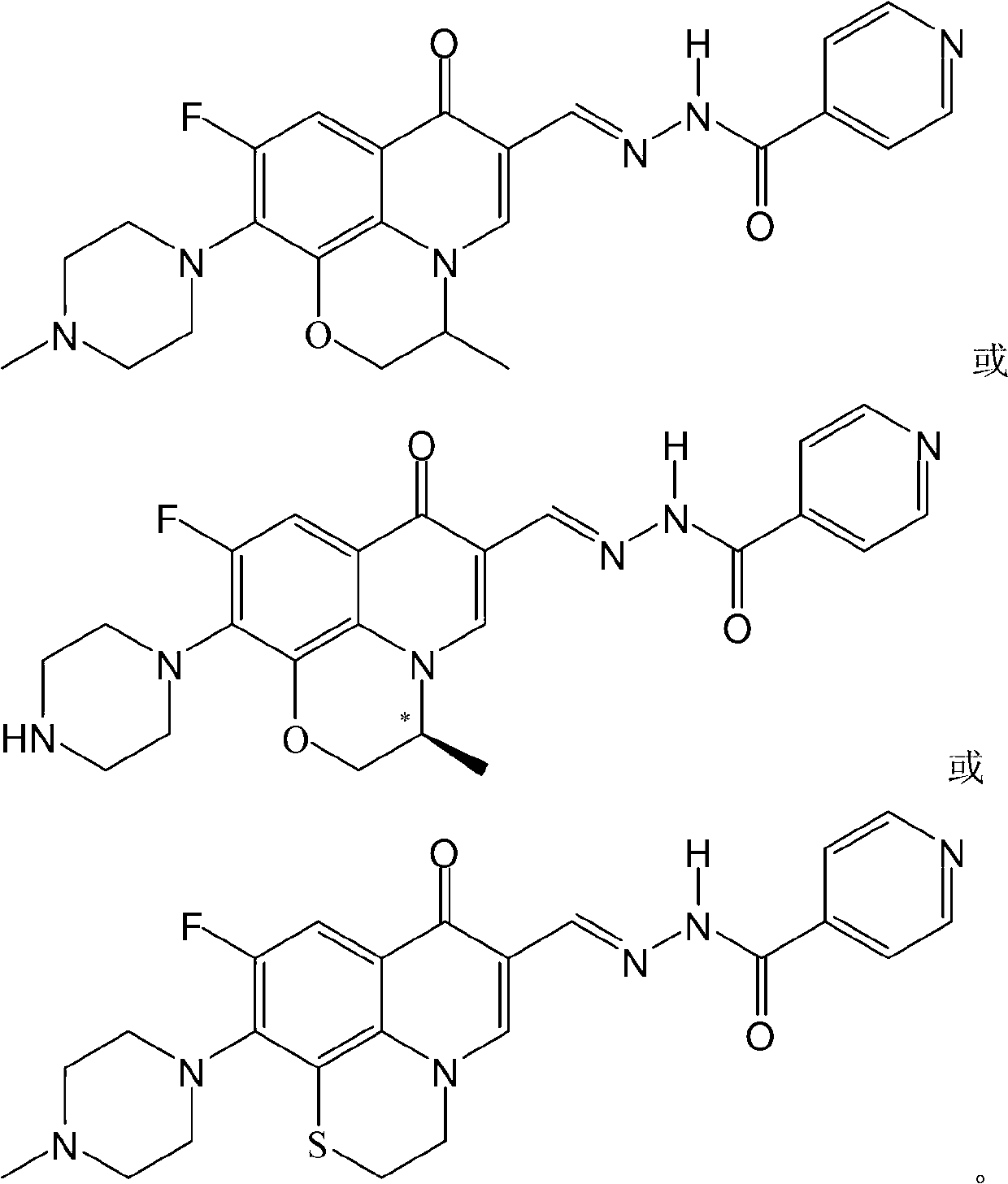
ActiveCN105524058AEnhanced inhibitory effectGood in vitro anti-tuberculosis activityAntibacterial agentsOrganic active ingredientsMulti-drug-resistant tuberculosisMinimum inhibitory concentration
The invention discloses a pyrazolo[1, 5-a]pyridine compound with the structure characteristic shown in the formula (I) or its pharmaceutically acceptable salt, stereoisomer or prodrug molecule and a use thereof. The compound has good in-vitro anti-tubercle bacillus activity, has the minimal inhibitory concentration (MIC) less than 0.1 micrograms per milliliter and partial MIC of 0.01 micrograms per milliliter and has strong inhibition effects on a clinically sorted multi-drug-resistant tuberculosis (MDR-TB) strain. In an in-vivo experiment, at a dosage of 20mg / kg / d, the pyrazolo[1, 5-a]pyridine compound can effectively eliminate H37Ra infection in a mouse and is a novel anti-tuberculosis compound.
Owner:GUANGDONG GOOD MEDICINE & HEALTH TECH CO LTD
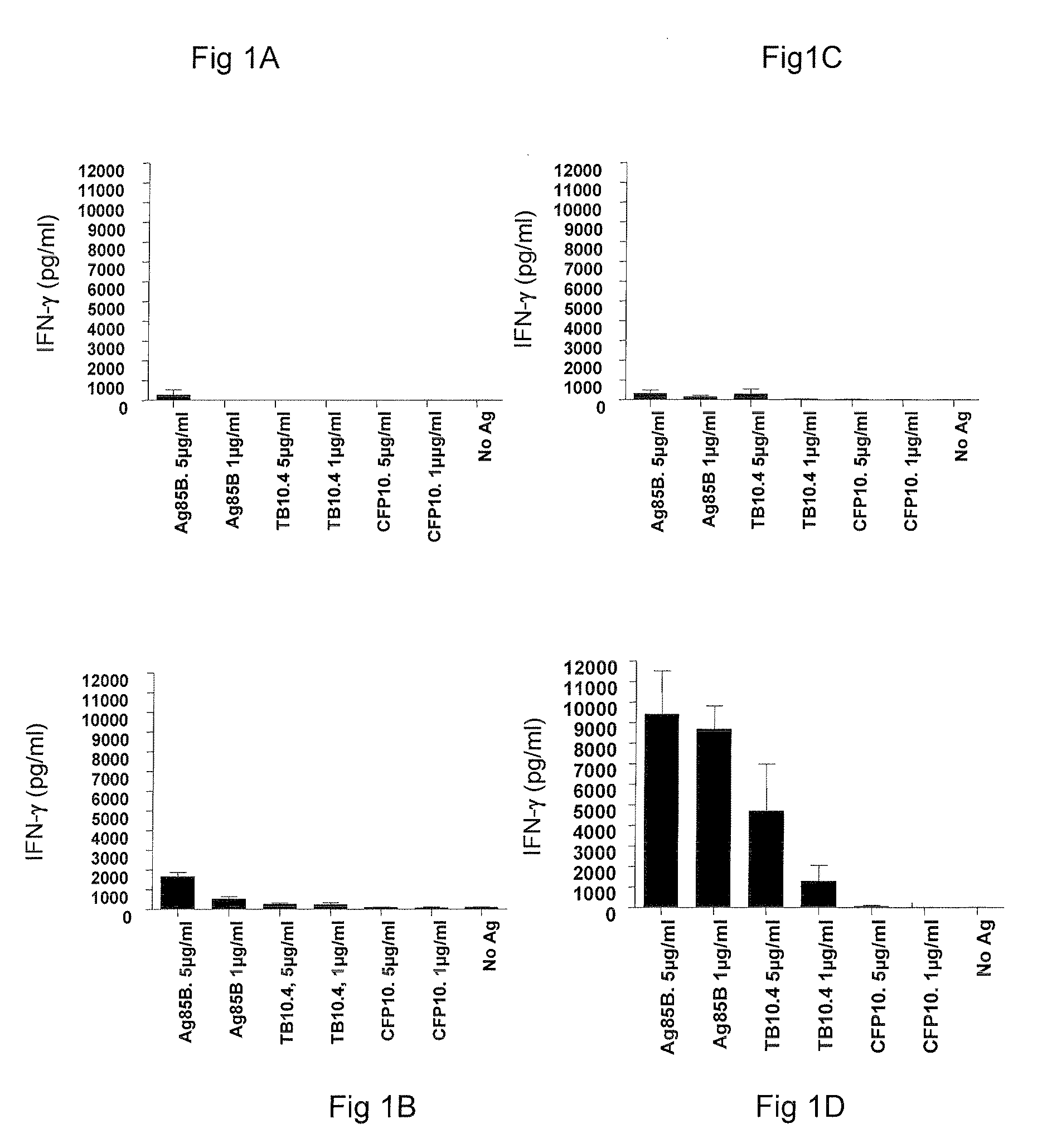
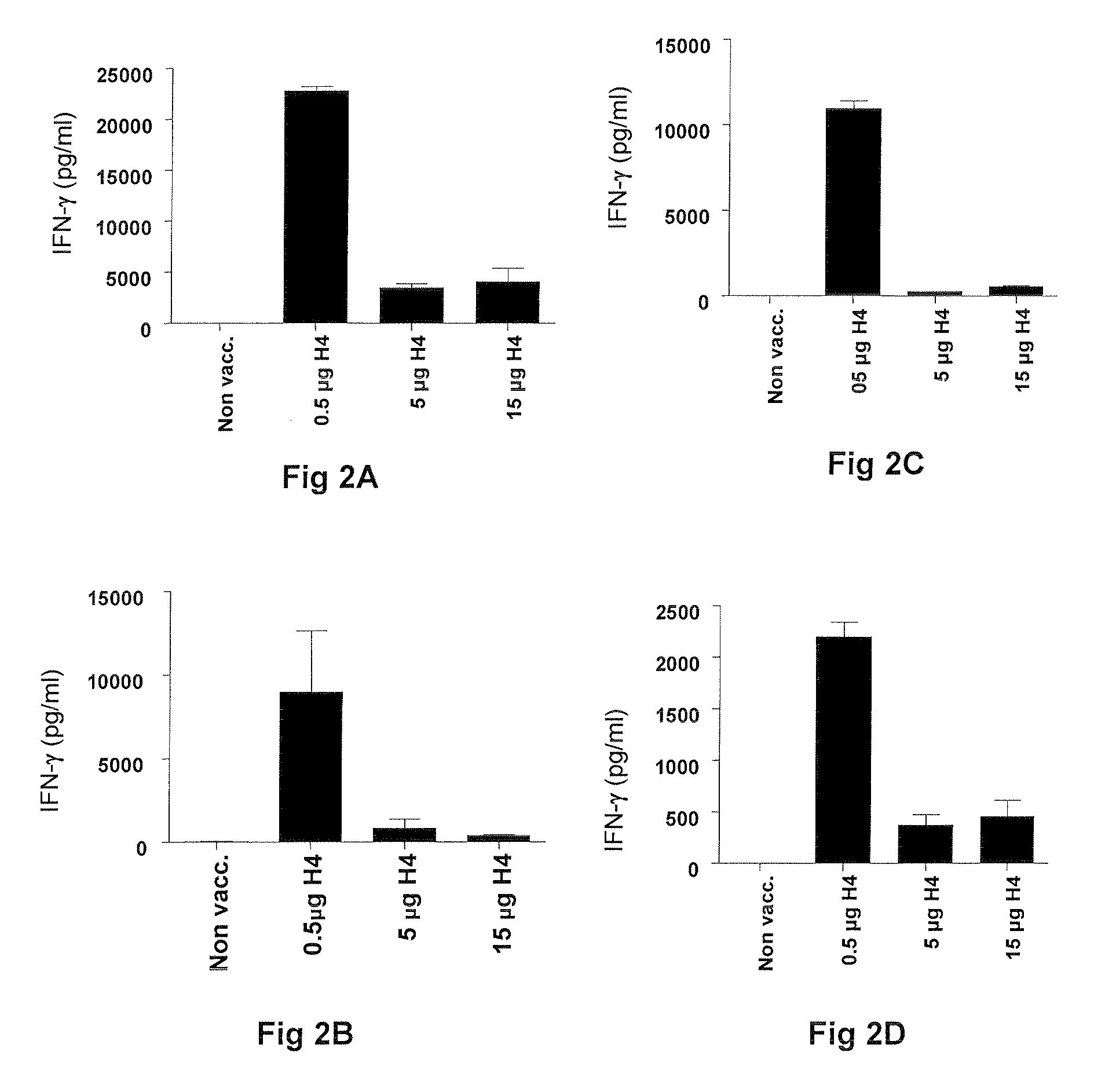
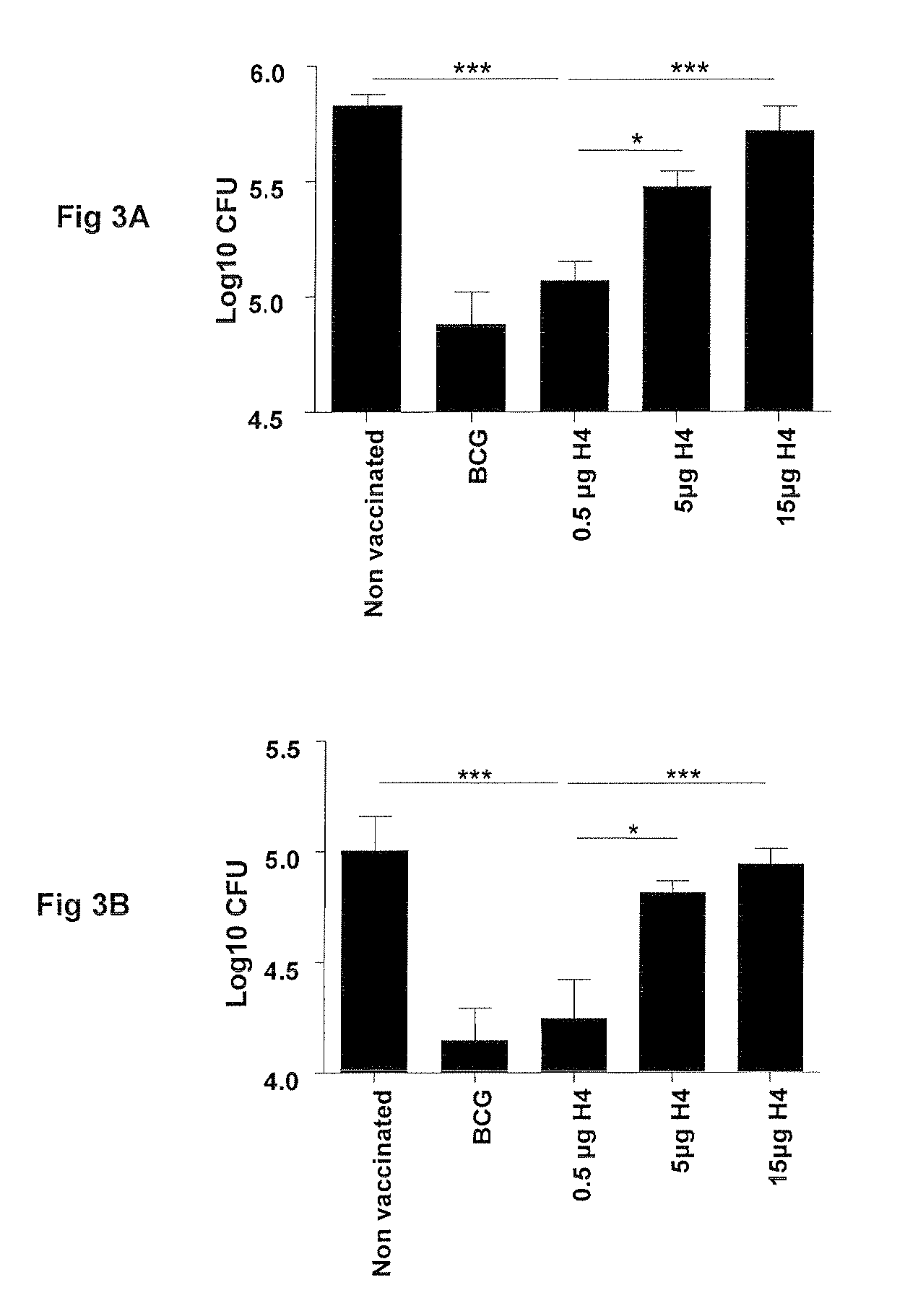
Vaccines comprising tb 10.4
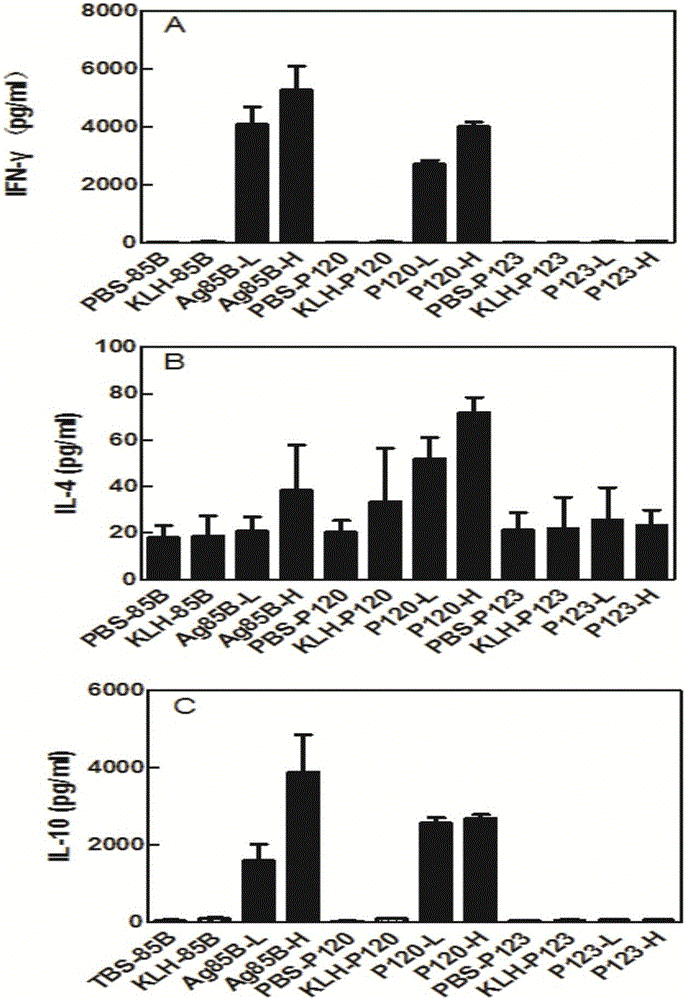
Vaccination with the combination of Ag85B-TB10.4 and IC31® adjuvant generated a high amount of polyfunctional CD4+T cells expressing high levels of IFN-γ, TNF-α, and IL-2. This in turn led to significant protection against infection with M. tuberculosis in the mouse aerosol challenge model of tuberculosis. Both the immunogenicity of the vaccine and its ability to protect against TB infection was highly dependent on the antigen dose. Thus, whereas the standard antigen dose of 5 μg, as well as 15 μg, did not induce significant protection against M. tuberculosis, reducing the dose to 0.5 μg increased both the immunogenicity of the vaccine as well as its protective efficacy to a level comparable to that observed in BCG vaccinated mice. Thus, the IC31® adjuvant, with the specified antigen dose, can induce a strong protective Th1 response against M. tuberculosis.
Owner:STATENS SERUM INST
Nitro imidazole compound, its preparation method and application
The invention relates to a nitro imidazole compound, its preparation method and use, in particular to a novel nitro imidazole compound, and its pharmaceutically acceptable salt, preparation method and application in preparing medicines for treating mycobacterium tuberculosis infectious diseases, especially infectious diseases caused by multidrug resistant mycobacterium tuberculosis. The nitro imidazole compound or the pharmaceutically acceptable salt thereof disclosed in the invention has good activity against mycobacterium tuberculosis in vitro, especially has strong activity against multidrug resistant mycobacterium tuberculosis.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Slow released compound antituberculotic preparation
The slow released compound antituberculotic preparation contains at least one of rifampicin, pyrazinamide, kanamycin, isoniazide, rifapentine, etc. The slow released preparation is slow released injection or slow released implanting agent. The slow released injection consists of slow released microsphere and solvent, the slow released microsphere contains slow releasing supplementary material and antituberculotic, and the solvent is special solvent containing suspending agent carboxymethyl cellulose sodium and of viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is EVAc, PLA, PLGA, sebacic acid copolymer, etc. The slow released compound antituberculotic preparation is set or injected into local tuberulosis focus to treat various kinds of intractable tuberulosis, and has medicine releasing period up to 30-40 days, less systemic toxicity and unique curative effect.
Owner:JINAN SHUAIHUA PHARMA TECH
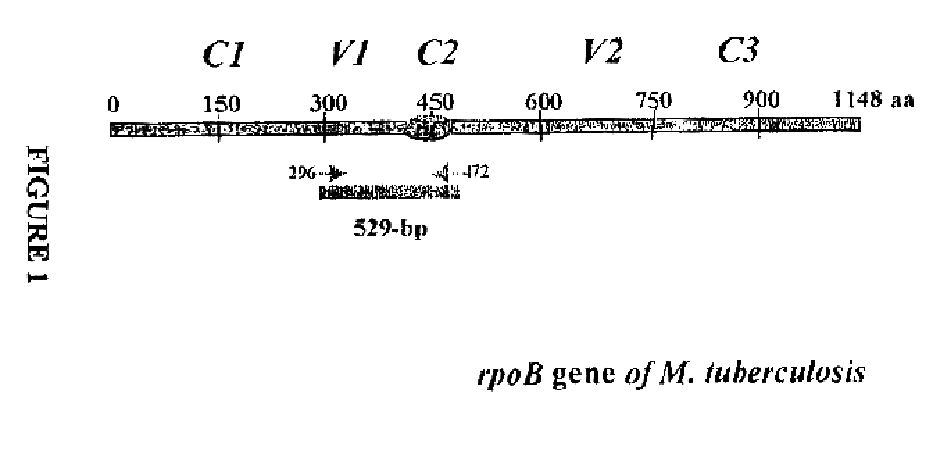
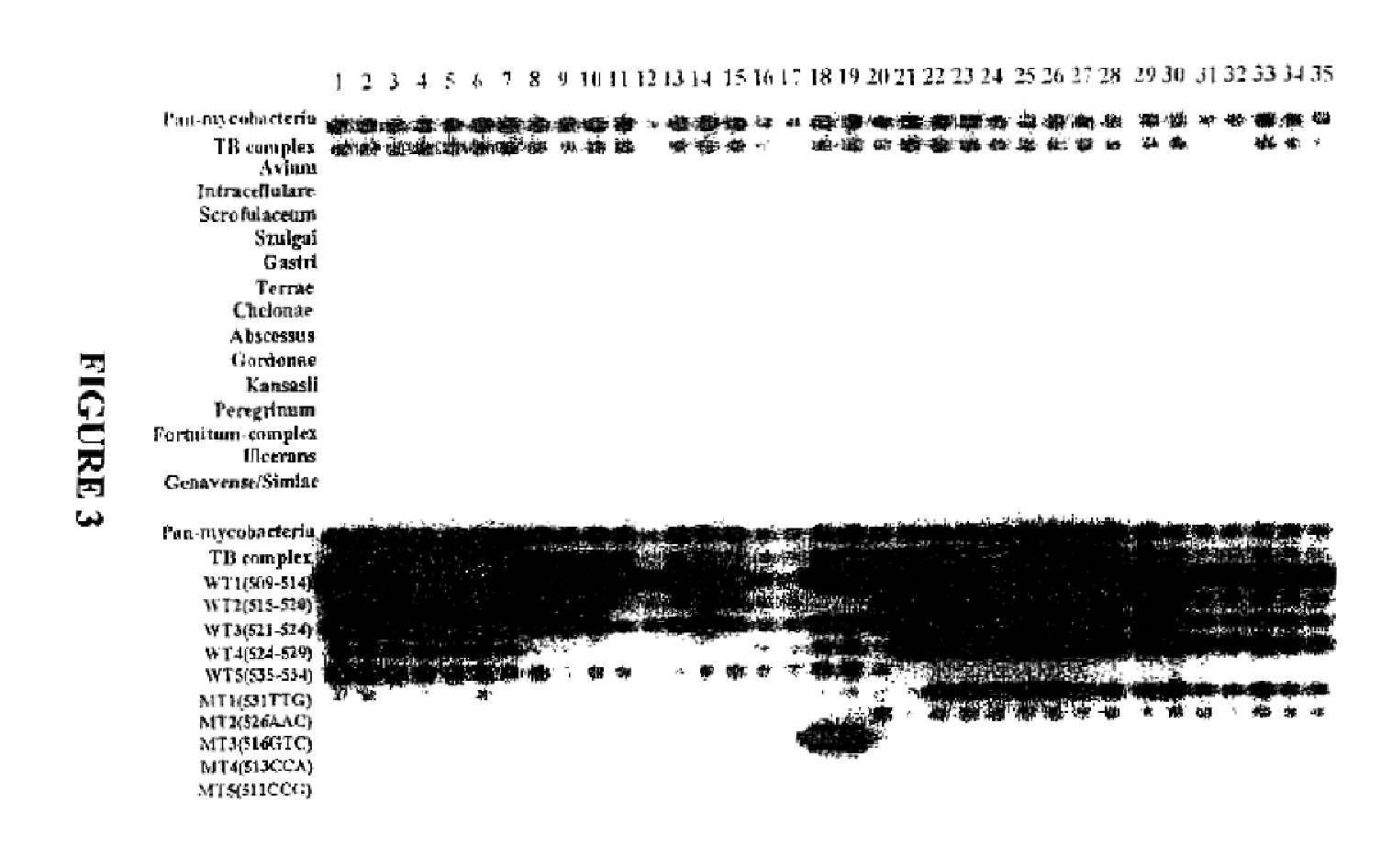
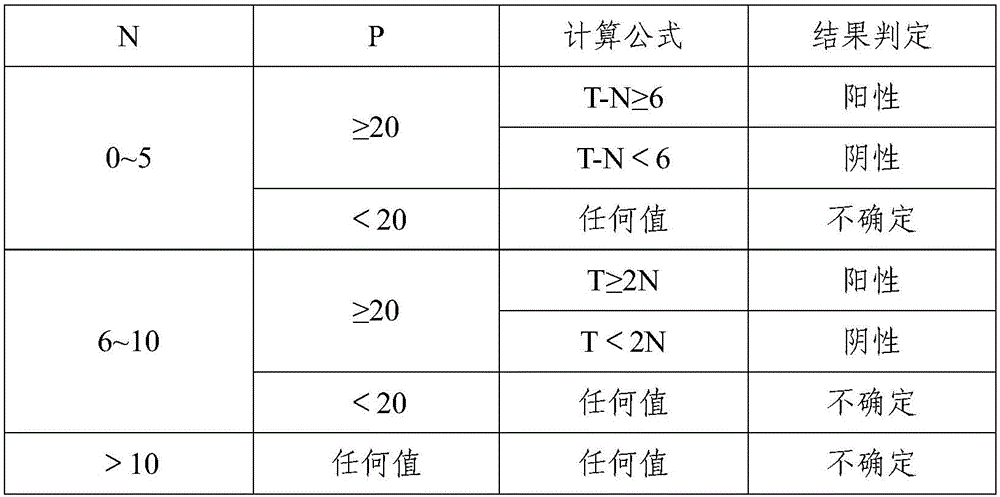
Method for identifying mycobacterium tuberculosis and mycobacteria other than tuberculosis, together with detecting resistance to an antituberculosis drug of mycobacteria obtained by mutation of rpob gene
The present invention provides a method for identifying Mycobacterium tuberculosis and non-tuberculosis Mycobacterium (MOTT), and for the determination of drug susceptibility of M. tuberculosis based on detection of mutations in the rpoB gene.
Owner:LEE HYEYOUNG +1
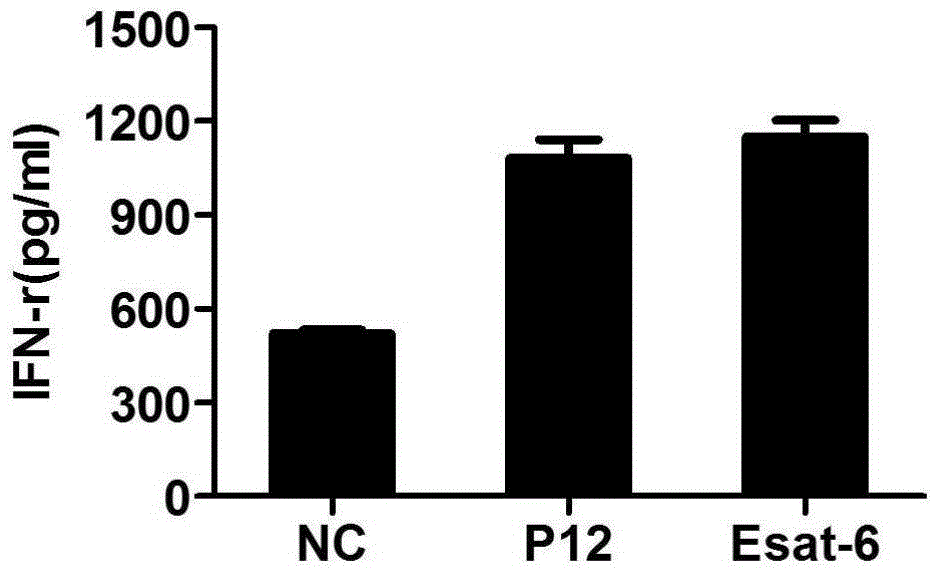
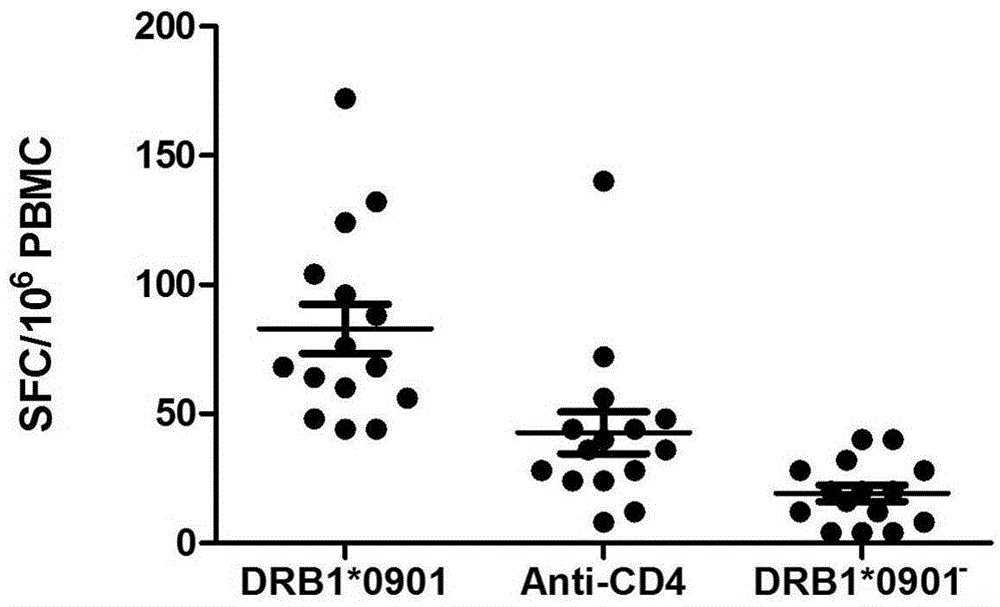
Mycobacterium tuberculosis specific CD4+T cell epitope peptide P12 and application thereof
InactiveCN105481947AHigh affinityAntibacterial agentsBacterial antigen ingredientsGenotypeTGE VACCINE
The present invention discloses a Mycobacterium tuberculosis specific CD4+T cell epitope peptide P12 and application thereof. The present invention comprehensively utilizes bioinformatics and immunoinformatics technologies, screens epitope peptide with anti-TB activity by using the antigen of Mycobacterium tuberculosis; the screened epitope peptide P12 and HLA-DRB1*09: 01 molecules have high affinity; and the epitope peptide can be widely identified by CD4+T cells of patients with TB. Since HLA-DRB1*09:01 genotype is widely carried by Chinese people, the Mycobacterium tuberculosis specific HLA-DRB1*09:01 restricted Th1 epitope peptide is not found currently. The determination of epitope peptide P12 provides a theoretical basis and a novel solution for the development of TB vaccines, diagnostic reagents and therapeutic drugs, and is of great significance for the prevention and treatment of tuberculosis.
Owner:SOUTHERN MEDICAL UNIVERSITY
Mycobacterium tuberculosis medicament sensitive phenotype detection method and application of method
InactiveCN102146430AWon't spreadReduce workloadMicrobiological testing/measurementBiotechnologyResazurin
The invention discloses a mycobacterium tuberculosis medicament sensitive phenotype detection method, which comprises the following steps of: 1) adding 2.5 to 6mul of 0.2 percent of resazurin dissolved by using methanol with mass percentage concentration into the inner side of a tube cover of a sterile centrifuge tube, and sterilizing the centrifuge tube after the methanol is volatilized; 2) inoculating 5 to 50mg of mycobacterium tuberculosis to be detected into a 7H9-S culture medium, regulating the concentration of the bacteria solution by adopting turbidimetry, regulating the concentration of the bacteria solution to the concentration of a Mcburney turbidimetric 1 tube by using sterile water or 0.9-percent physiological saline, and diluting the bacteria solution by 20 times by using the 7H9-S culture medium; 3) inoculating 50 to 150mu of diluted bacteria solution into the centrifuge tube of the step 1); 4) adding 100mul of anti-tubercular medicament solution diluted by 7H9-S and to be detected into the centrifuge tube of the step 3), wherein the concentration of the medicament to be detected is 0.01mug / ml to 2.5mg / ml; 5) after the centrifuge tube of the step 4) is covered closely, culturing the solution for 6 to 7 days at the temperature of 37 DEG C; and 6) inverting and oscillating the centrifuge tube of the step 5), and culturing the solution for 12 to 48 hours at the temperature of 37 DEG C after the solution becomes blue. Proved by experiments, in the mycobacterium tuberculosis medicament sensitive phenotype detection method and application of the mycobacterium tuberculosis medicament sensitive phenotype detection method in anti-tubercular medicament screening, the method has low workload and short detection time; compared with a traditional L-J medium medicament sensitive detection method, the accuracy reaches 90 to 99 percent; and the method has good biological safety, does not cause laboratory propagation of tuberculosis, is suitable for large-scale.
Owner:LANZHOU UNIVERSITY
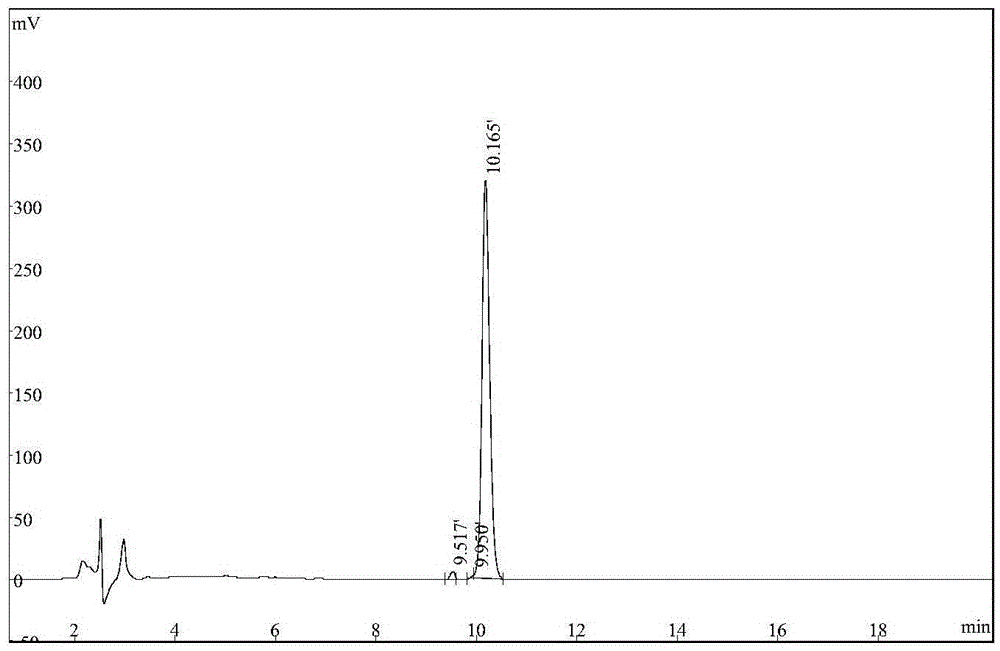
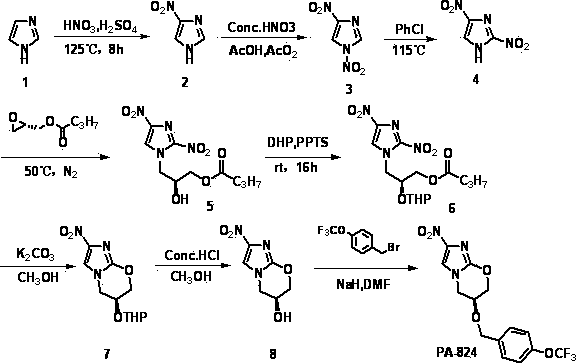
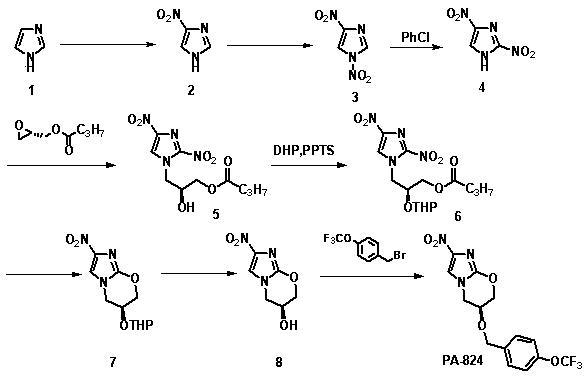
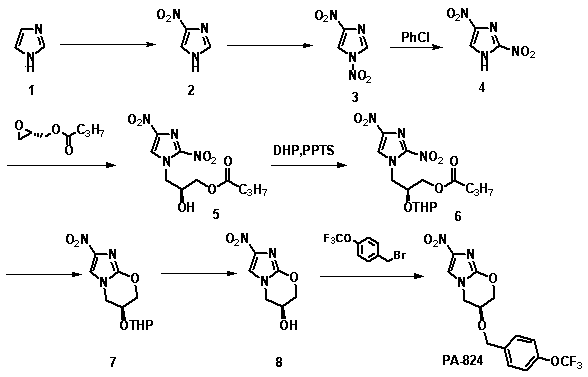
Synthetic method of anti-tuberculosis candidate drug PA-824
InactiveCN104177372AEasy to operateRaw materials are easy to getOrganic chemistryBenzoyl bromidePharmaceutical drug
The invention relates to a synthetic method of an anti-tuberculosis candidate drug PA-824. The method involves an eight-step reaction and comprises the following steps: firstly obtaining a key intermediate compound 8 and then reacting with 4-trifluoromethoxy benzyl bromide to obtain the final product PA-824. The entire reaction has the advantages of easily available raw materials, simplicity in operation, convenience in purification and easiness in scale production. The compound 8 is as shown in the specification.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
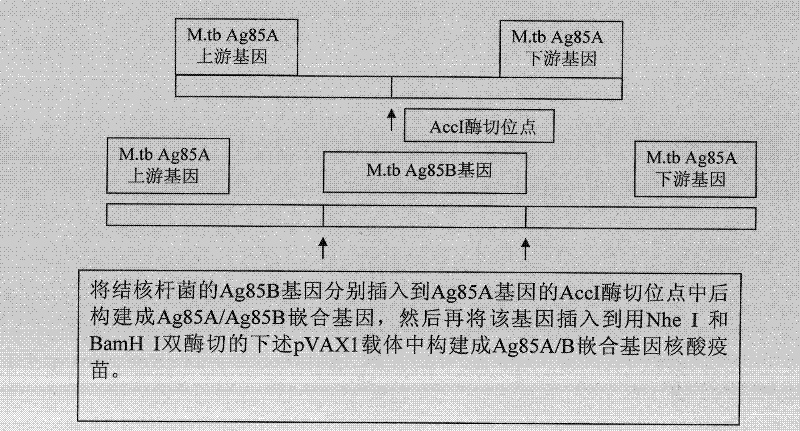
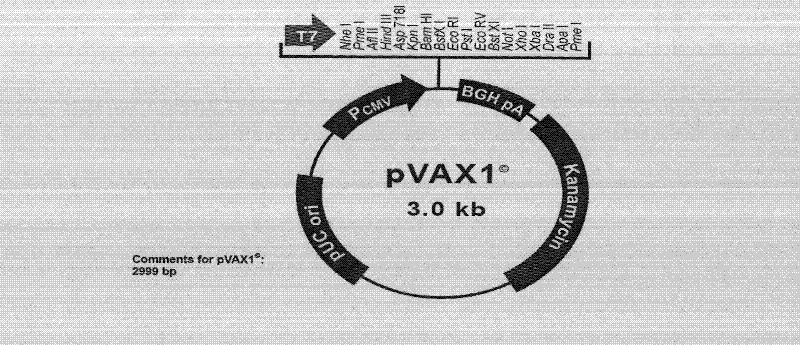
Mycobacterium tuberculosis ag85ab chimeric gene vaccine, its preparation method and application
ActiveCN102268446AStrong immune responseGood treatment effectAntibacterial agentsBacterial antigen ingredientsAdjuvantLevamisole
The invention provides a mosaic gene, which comprises a gene for coding tubercle bacillus protein Ag85a and a gene which is mosaiced at a Kpn I recognition sequence at sites 245-250 and / or an Acc I recognition sequence at sites 430-435 of Ag85a gene for coding the tubercle bacillus protein Ag85b. The invention provides a mosaic gene-containing mosaic tubercle bacillus gene vaccine, wherein the Ag85a gene is connected to a eukaryotic expression vector. The invention also provides a preparation method of the mosaic tubercle bacillus gene vaccine. The method comprises the following steps of: performing PCR (Polymerase Chain Reaction) amplification on an Ag85b gene segment; inserting the amplified Ag85b gene segment into the Ag85b gene-containing eukaryotic expression vector; and connecting by using ligase. The mosaic tubercle bacillus gene vaccine can be used for treating drug-resistant tubercle bacillus infection and can also be used in combination with levamisole serving as an adjuvantto induce stronger anti-tubercular cell immunological response.
Owner:GUAN DINGTAI HAIGUI BIOLOGICAL TECH CO LTD
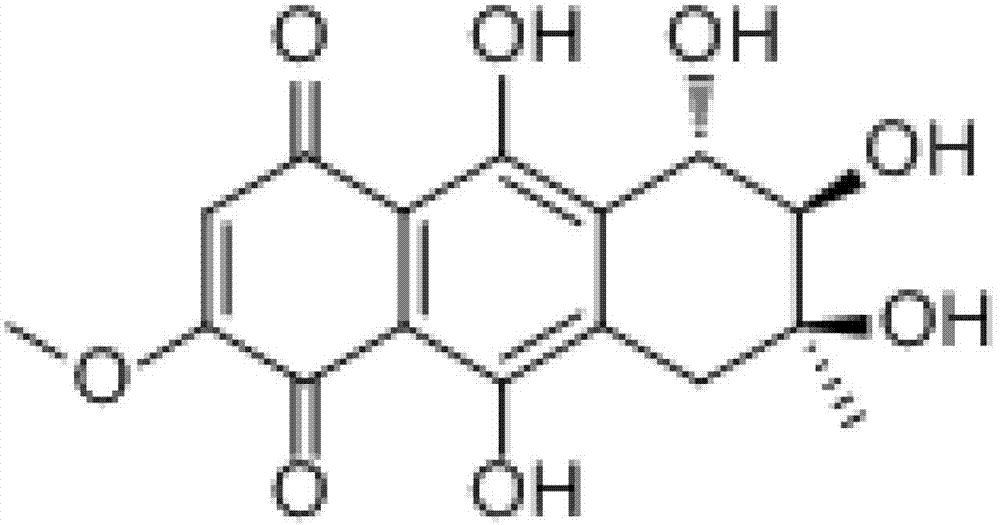
Uridine peptide antibiotic, pharmaceutically acceptable salt, producing method and uses thereof
The present invention provides antibiotics of urinate glucoside peptide category with a structural formula (I) and the acceptable salt in pharmaceutics. The antibiotics can be prepared through fermentation and cultivation of produced fungus. The compound of the present invention is a group of cell-wall peptidoglycan synthesized inhibitor, has anti-tuberculosis activity and pyocyanic fungus, and can be used for preparing anti-infective drugs.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
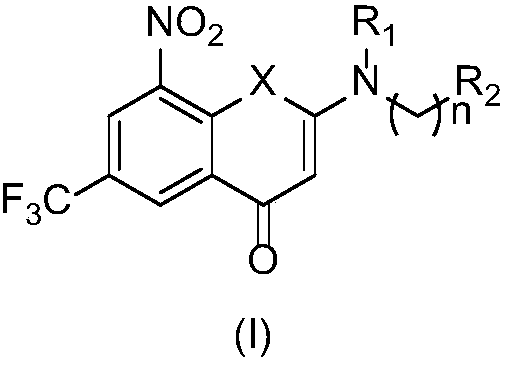
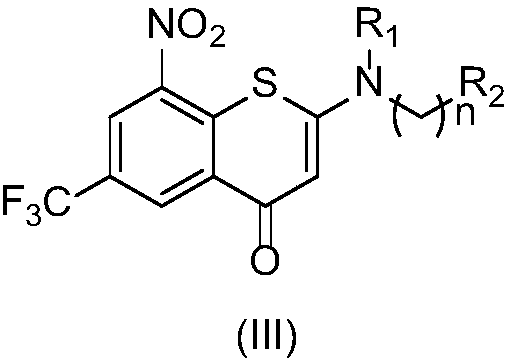
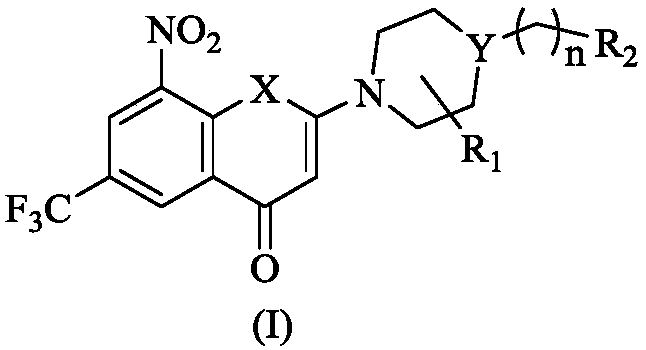
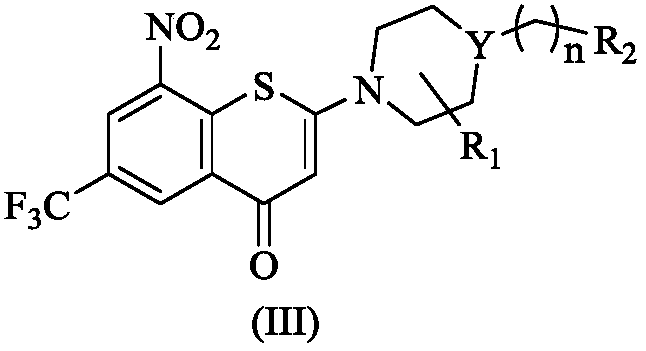
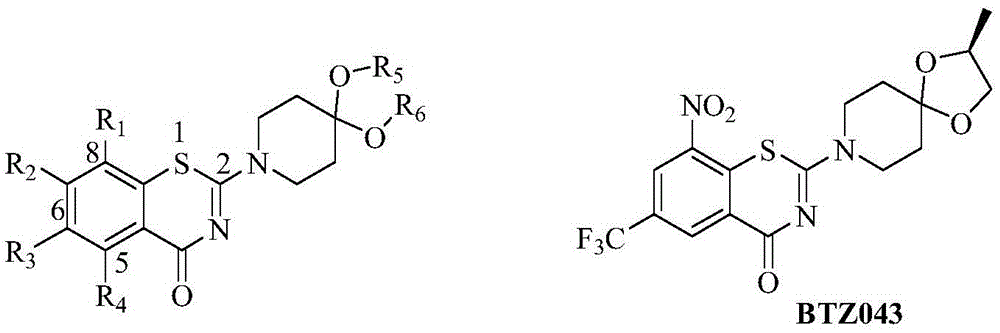
2-substituted amino-5-trifluoromethyl-8-nitrobenzo(thio-)pyran-4-one compound, preparation method therefor and use of 2-substituted amino-5-trifluoromethyl-8-nitrobenzo(thio-)pyran-4-one compound
The invention discloses a 2-substituted amino-5-trifluoromethyl-8-nitrobenzo(thio-)pyran-4-one compound represented by a formula (I) shown in the description, a preparation method therefor and application of the 2-substituted amino-5-trifluoromethyl-8-nitrobenzo(thio-)pyran-4-one compound in drugs for treating and / or preventing infectious diseases induced by mycobacterium tuberculosis. Specifically, the invention relates to the compound represented by the formula (I) and pharmaceutically-acceptable salts thereof and a pharmaceutical composition containing the compound disclosed by the invention, wherein X, R1, R2 and n are as defined in the description. The invention aims at preparing a novel compound with the activity of resisting the mycobacterium tuberculosis; and as a potential new drug, the compound can be applied to the treatment or prophylactic treatment of microbial infectious diseases, particularly pulmonary tuberculosis (TB) induced by mycobacteria, and meanwhile, the compound can be used for overcoming problems related to drug resistance of the mycobacterium tuberculosis.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
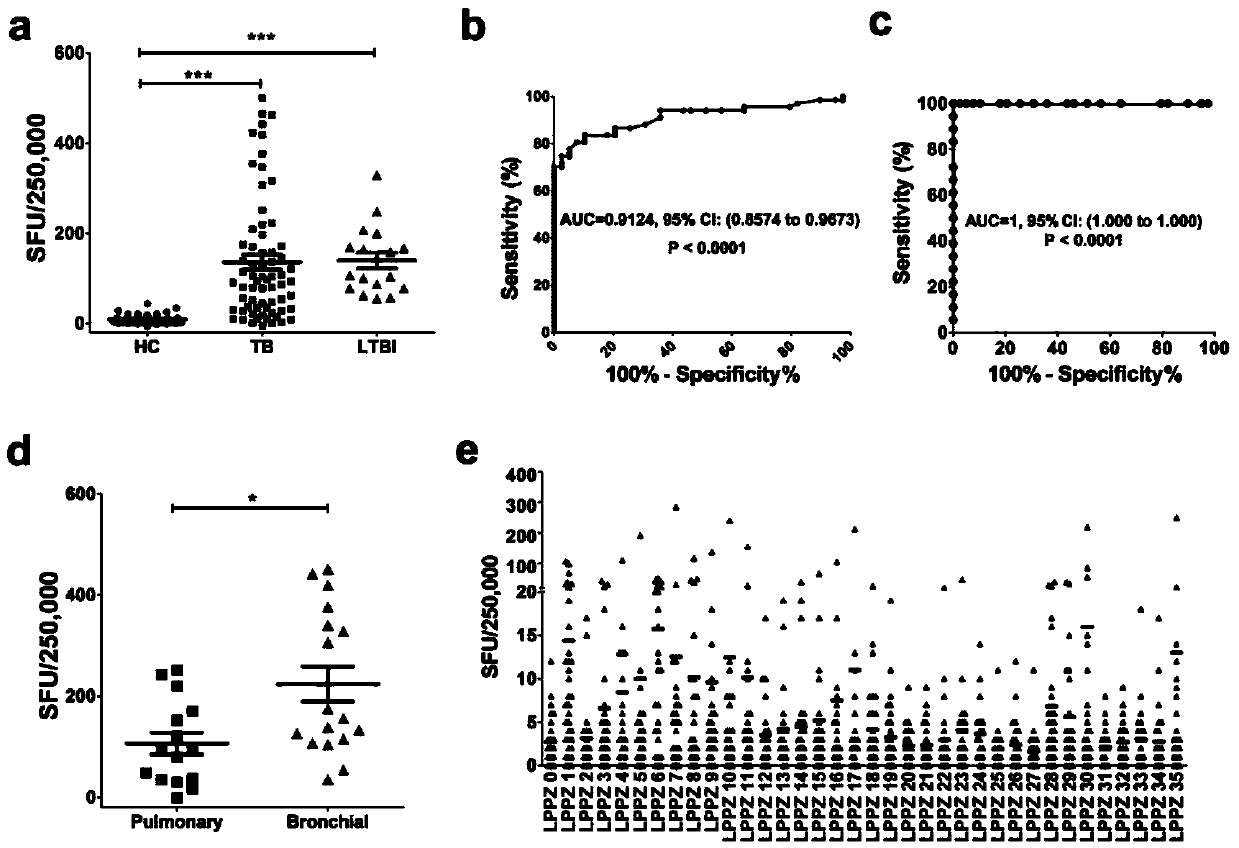
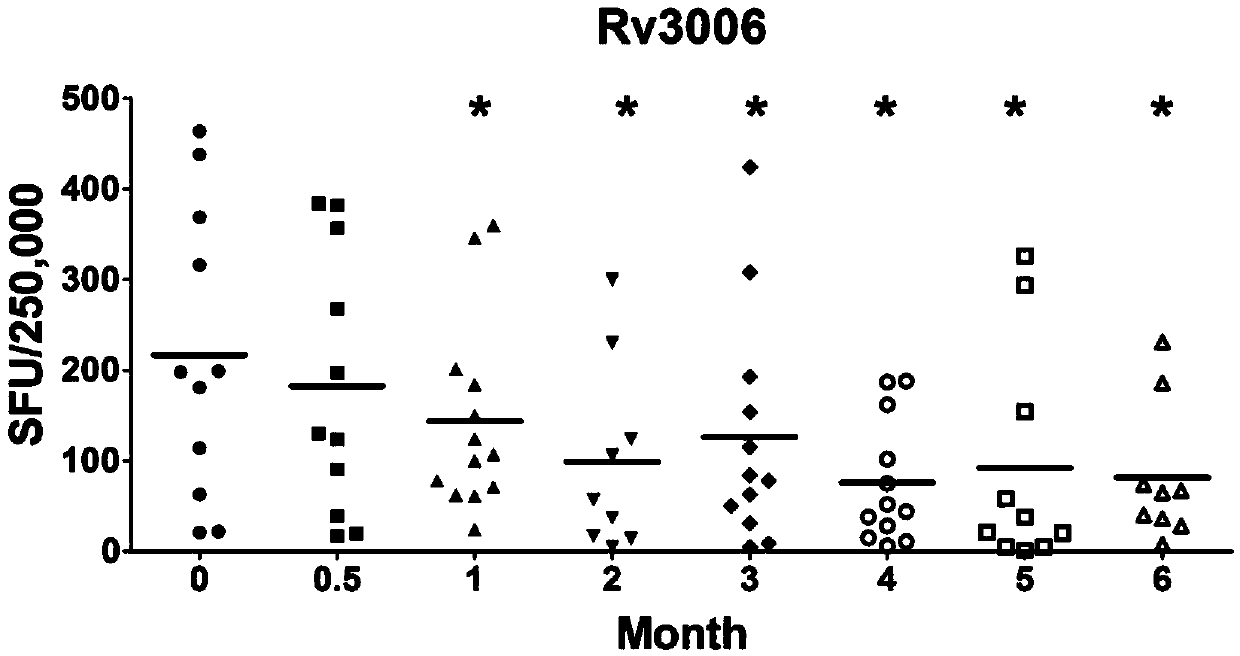
Reagent for mycobacterium tuberculosis infection detection, clinical treatment effect tracking and antituberculous vaccine development and application thereof
InactiveCN104004069AGood tracerGood diagnostic value for tuberculosisAntibacterial agentsBacterial antigen ingredientsAntigenMycobacterium Infections
The invention discloses a reagent for mycobacterium tuberculosis infection detection, clinical treatment effect tracking and antituberculous vaccine development. The novel mycobacterium tuberculosis derived reagent Rv3006 (LPPZ) prepared by a gamma-interferon gamma release assay and enzyme linked immunosorbent assay comprises antigen or polypeptide and analogues thereof, wherein terminal C to terminal N in the amino acid sequence of the Rv3006 antigen is as shown in SEQ ID NO:1. By utilizing the reagent, tuberculous patients in the active phase and tuberculous latent infectors can be well detected; the specific immune response of Rv3006 is reduced along with treatment development and disease condition remission, which indicates that the reagent can be used for well tracking the clinical treatment situation of a tuberculous patient, thus having a good clinical application prospect. Furthermore, the reagent has a protective effect on a mouse infected by a mycobacterium tuberculosis standard toxic strain H37Rv, thus having a good development prospect of antituberculous vaccines.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
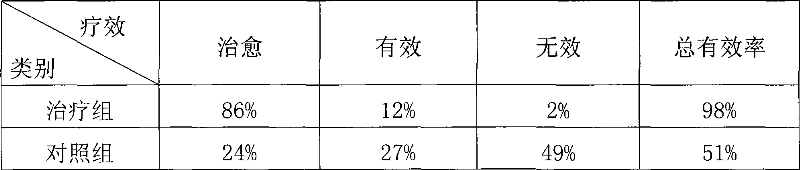
Medicinal composition for treating tuberculosis and its preparing process
InactiveCN101041024AImprove immunityAvoid the pain of surgeryAntibacterial agentsAmphibian material medical ingredientsMedicinal herbsToad Venom
Disclosed is a pharmaceutical composition for treating tuberculosis which is prepared from the following Chinese medicinal herbs (by weight ratio): Radix Ranunculi Ternati 1-3 weight parts, toad venoms 1-3 weight parts, rhubarb horsetails 1-3 weight parts, fritillary 1-3 weight parts, selfheal 1-3 weight parts, asparagus root 1-3 weight parts. The invention also discloses the process for preparing the pharmaceutical composition.
Owner:刘玉正
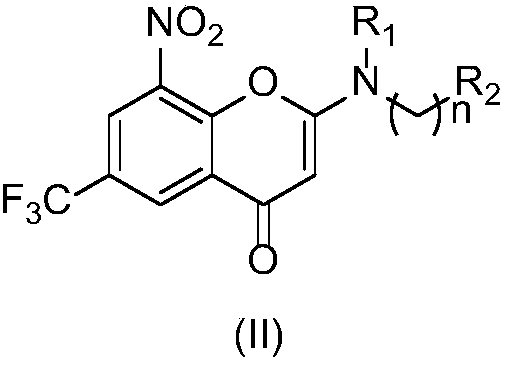
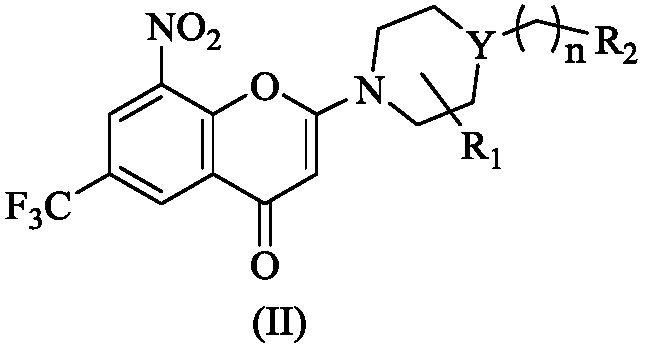
2-azacyclo-5-trifluoromethyl-8-nitrobenzo(thio)pyranyl-4-one compound
The invention discloses a 2-azacyclo-5-trifluoromethyl-8-nitrobenzo(thio)pyranyl-4-one compound, a preparation method thereof, and an application of the compound in a medicine for preventing and / or treating infectious diseases caused by mycobacterium tuberculosis. Specifically, the invention relates to a compound shown in the formula (I), an isomer of the compound, a pharmaceutically acceptable salt of the compound, and a pharmaceutical composition containing the compound, wherein X, Y, R<1>, R<2> and n are as described in the specification. The invention aims to prepare the novel compound with anti-mycobacterium tuberculosis activity, and the novel compound is used as a potential new medicine, can be used for treating or prophylactically treating infectious diseases caused by bacteria, especially tuberculosis (TB) caused by mycobacterium, and can also be used for overcoming the problems related to drug resistance of mycobacterium tuberculosis.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
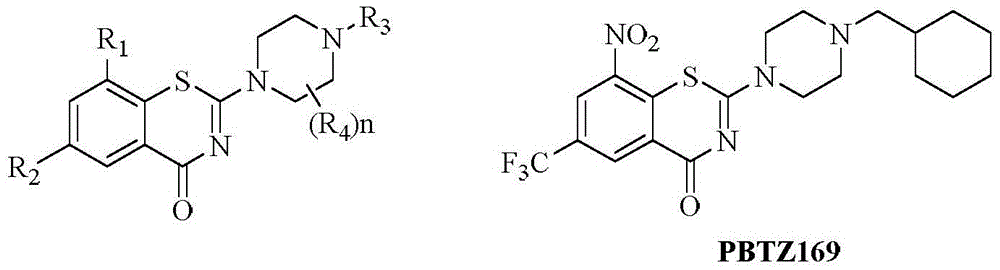
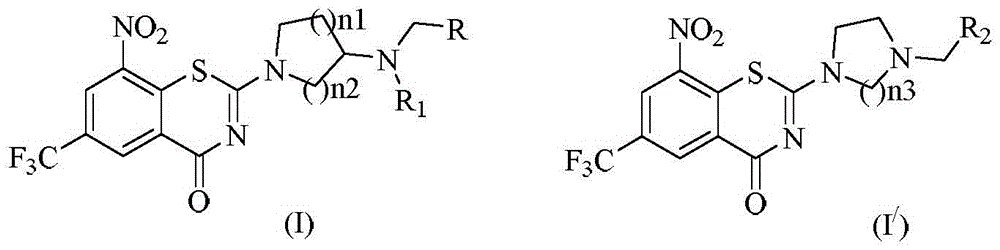
Benzothiazine-4-ketone compounds containing basic nitrogen heterocyclic fragments and preparing methods of benzothiazine-4-ketone compounds
ActiveCN105669664AHigh activityGood curative effectAntibacterial agentsOrganic active ingredientsBenzeneNitrogen
The invention relates to benzothiazine-4-ketone compounds (shown in the formula I and formula I' in the description) containing basic nitrogen heterocyclic fragments, preparing methods and medical application of the benzothiazine-4-ketone compounds and antituberculosis drug compositions with the benzothiazine-4-ketone compounds as effective constituents, in particular to a 6-trifluoromethyl-8-nitryl-4H-benzo[e][1,3] thiazine-4-ketone compound. A 2-substituent group is 1-nitrogen heterocyclic alkyl or dinitrogen heterocyclic alkyl. R represents H, an alkyl group of 1-4 C atoms, heterocyclic alkyl of 4-7 C atoms, a phenyl group and a substituted phenyl; R1 represents H, an alkyl group of 1-3 C atoms and heterocyclic alkyl of 3-6 C atoms; R2 represents H, an alkyl group of 1-4 C atoms, heterocyclic alkyl of 4-7 C atoms, a phenyl group and substituted phenyl; n1 represents 0-1; n2 represents 1-3; n3 represents 1 and 3.
Owner:ZHEJIANG STARRY PHARMA +1
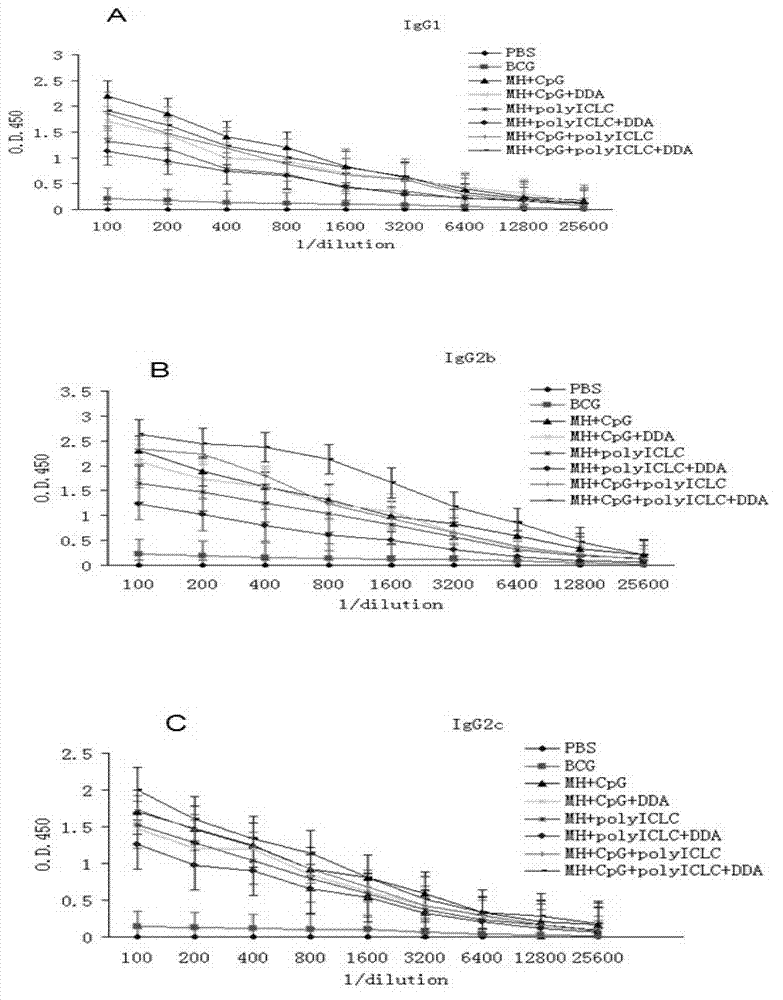
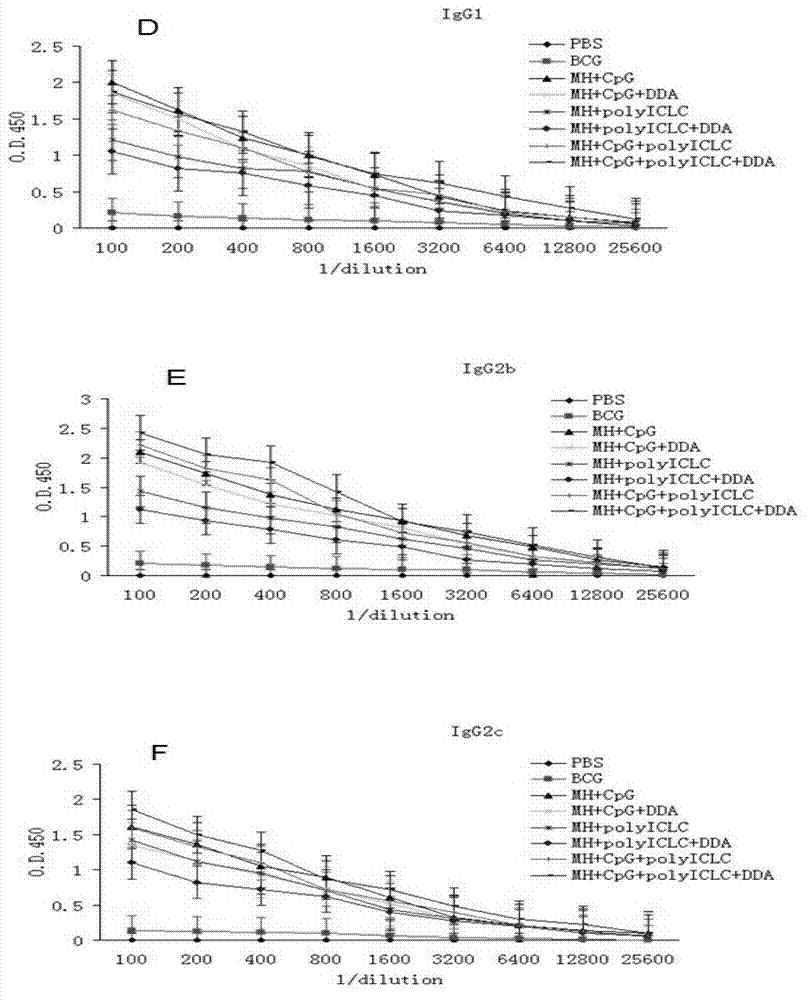
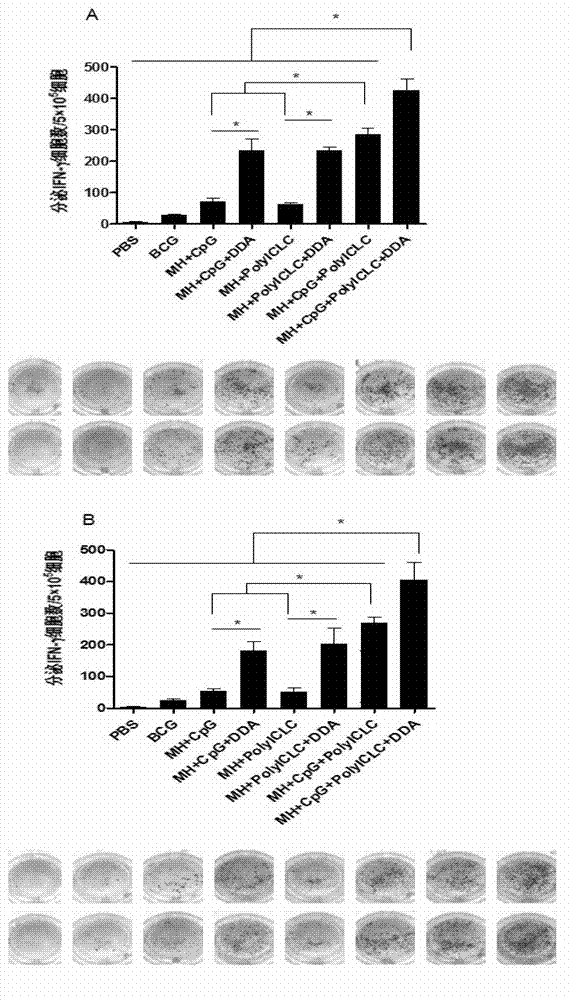
Compound adjuvant for tuberculosis subunit vaccine, tuberculosis subunit vaccine from same and preparation method and application thereof
InactiveCN103203018AImproving immunogenicityHelps induce Th1 type anti-TB immune responseAntibacterial agentsBacterial antigen ingredientsAdjuvantNucleotide
The present invention discloses a compound adjuvant for tuberculosis subunit vaccine, containing cationic liposome dimo-thylidioctyl ammonium bromide (DDA), TLR9 ligand CpG oligodeoxynucleotides 2395 and TLR3 ligand PolyICLC, and also provides the tuberculosis subunit vaccine and preparation method and application thereof. The beneficial effect of the invention is that the compound adjuvant for tuberculosis subunit vaccine provided by the present invention enhances the immunogenicity of the fusion protein of Mycobacterium tuberculosis, contributing to the induction of the tuberculosis fusion protein to Th1-type anti-tuberculosis immune response.
Owner:LANZHOU UNIVERSITY
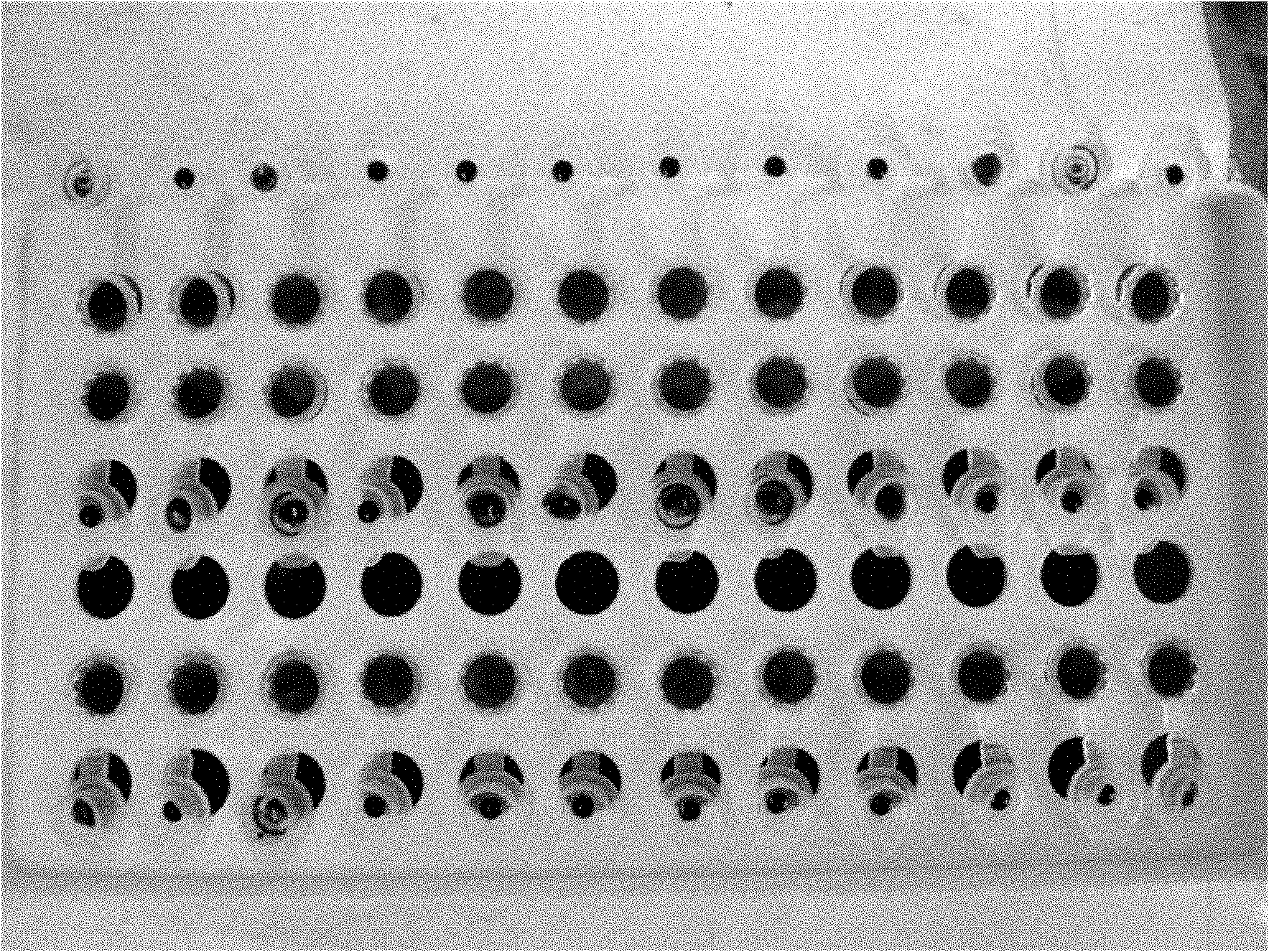
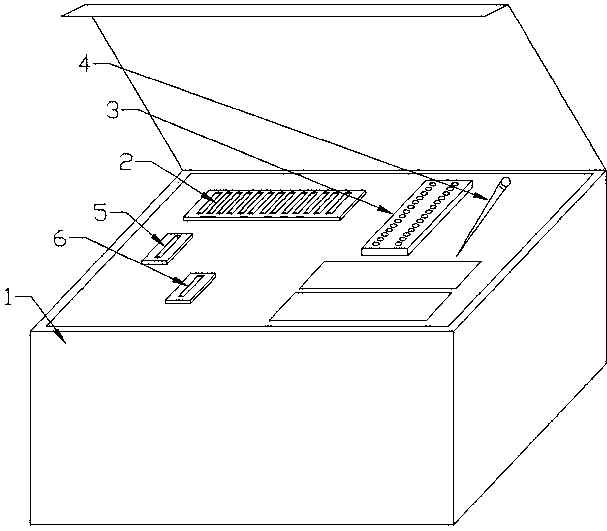
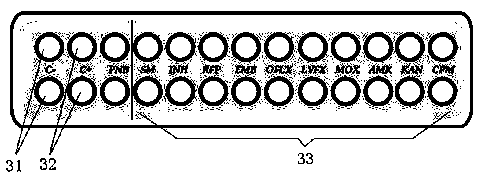
Mycobacterium drug sensitive detection kit and testing method thereof
InactiveCN103740802AReduce generationEasy to operateMicrobiological testing/measurementTuberculosis mycobacteriumAntituberculosis drug
The invention discloses a mycobacterium drug sensitive detection kit which is safe and effective, convenient to operate, short in testing time and wide in testing drug range and a testing method thereof. The mycobacterium drug sensitive detection kit provided by the invention comprises a kit body (1), wherein the kit body (1) internally comprises a plurality of drug sensitive culture mediums (2), a drug sensitive testing plate (3), a sterile suction nozzle (4), a sterile diluent (5), an infectious microbe inhibitor (6), a specification and a report label, the drug sensitive culture medium (2) comprises a basal culture medium, a growth promoter and a bacteriostatic agent, and a negative control hole (31), a positive control hole (32) and a plurality of drug containing holes (33) are formed in the drug sensitive testing plate (3), wherein the drug containing holes (33) are arranged into a matrix and are coated with different antituberculosis drugs. The mycobacterium drug sensitive detection kit can be applied to the field of mycobacterium tuberculosis detection.
Owner:ZHUHAI ENCODE MEDICAL ENG
Applications of Mycobacterium tuberculosis antigen protein Rv0446c and T-cell epitope peptide thereof
ActiveCN106248934AReduce false positivesStrong immune responseAntibacterial agentsBacterial antigen ingredientsAntigenStimulant
The present invention relates to applications of Mycobacterium tuberculosis antigen protein Rv0446c and a T-cell epitope peptide thereof in preparation of tuberculosis detection reagents, vaccines and medicines, wherein the amino acid sequences of the antigen protein Rv0446c and the T-cell epitope peptide thereof are respectively represented by SEQ ID NO:1-5. According to the present invention, the Mycobacterium tuberculosis antigen protein Rv0446c and the T-cell epitope peptide thereof are used as the stimulants for the specific T cell and B cell immune response caused by Mycobacterium tuberculosis infection, and the false positive caused by the impure antigen can be reduced compared with the use of the complete antigen in the prior art; and the detection reagents prepared from the antigen protein Rv0446c and the T-cell epitope peptide thereof can be widely used for assisted diagnosis of tuberculosis, epidemiological surveillance and other related fields, and the tuberculosis vaccines and the anti-tuberculosis drugs prepared from the antigen protein Rv0446c and the T-cell epitope peptide thereof can be used for prevention and treatment of tuberculosis.
Owner:ICDC CHINA CDC
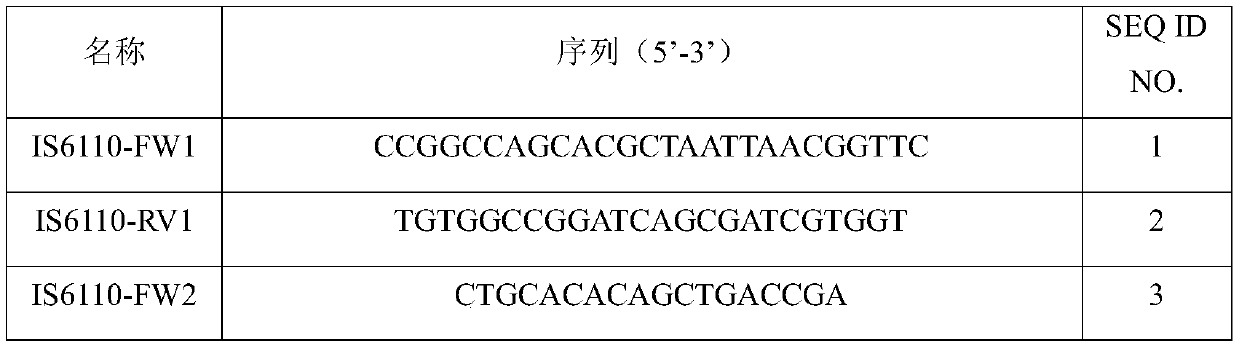
Tubercle bacillus drug tolerance detection reagent kit and tubercle bacillus drug tolerance detection method
PendingCN111172303AWide range of drug resistance detectionEasy constructionMicrobiological testing/measurementLibrary creationAntituberculosis drugTuberculosis bacillus
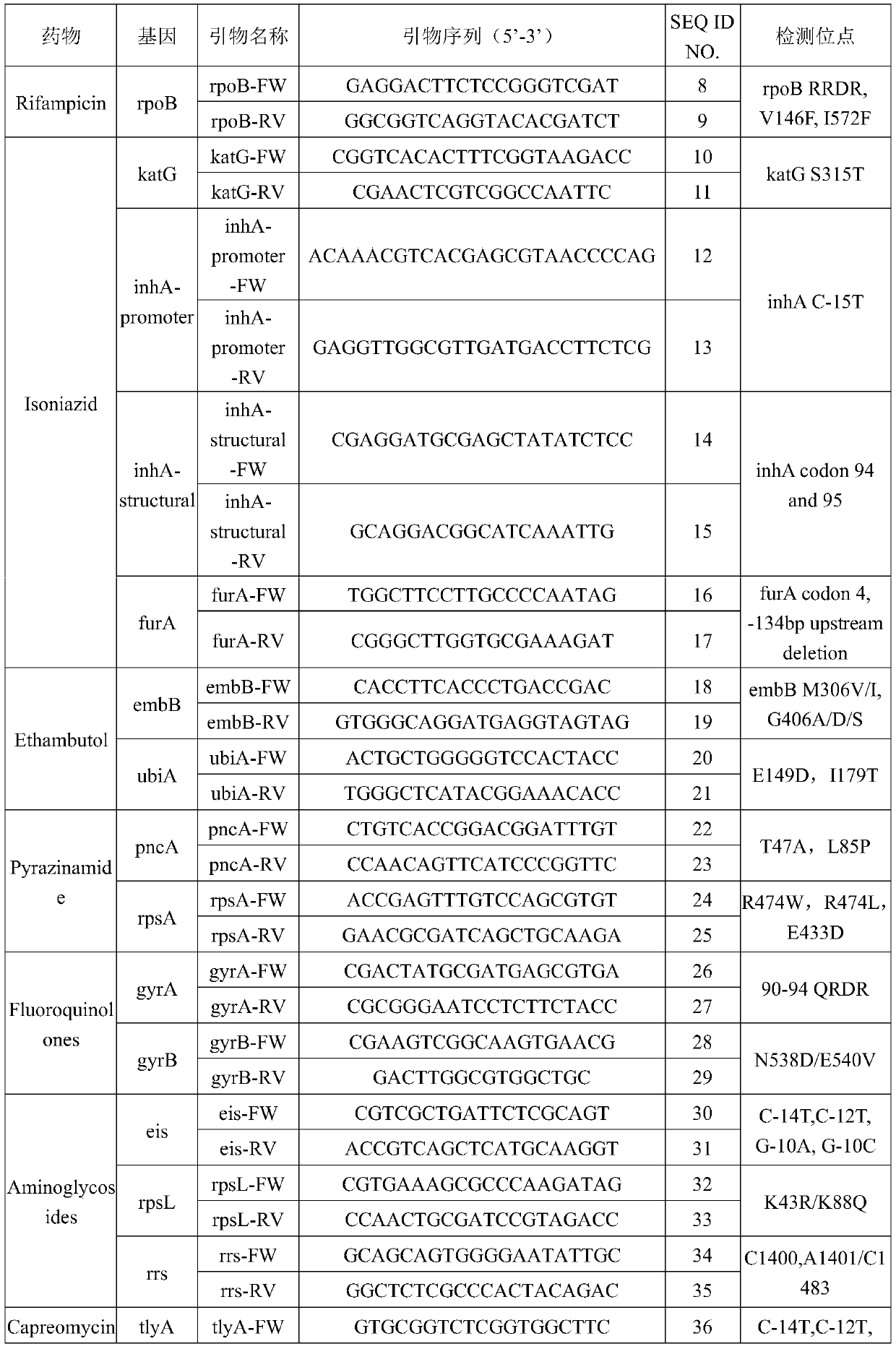
The invention provides a tubercle bacillus drug tolerance detection reagent kit and method. The tubercle bacillus drug tolerance detection reagent kit comprises a tubercle bacillus drug tolerance detection reagent, wherein the tubercle bacillus drug tolerance detection reagent comprises a sequencing primer in accordance with a tubercle bacillus drug tolerance gene; the tubercle bacillus drug tolerance gene comprises one or more genes of rpoB, katG, inhA-promoter, inhA-structural, furA, embB, ubiA, pncA, rpsA, gyrA, gyrB, eis, rpsL, rrs, tlyA, rplC and rrl; and further, the reagent kit also contains a tubercle bacillus nucleic acid detection reagent, and the tubercle bacillus nucleic acid detection reagent comprises a primer pair 1 in accordance with IS6110, a primer pair 2 in accordance with the IS6110 and a probe primer in accordance with the IS6110. Through the adoption of the tubercle bacillus drug tolerance detection reagent kit disclosed by the invention, tubercle bacillus nucleicacid in samples can be quickly detected, and positive samples can be further subjected to drug tolerance detection; and the tubercle bacillus drug tolerance detection reagent kit has good sensitivity, good specificity and good accuracy, and can perform mutation detection on 48 sites of 17 drug tolerance genes of common antituberculosis drugs and fragment deficiency detection of an intergenic region, so that the tuberculosis medication can be more accurately and comprehensively guided.
Owner:GUANGZHOU KINGMED DIAGNOSTICS GRP CO LTD +1
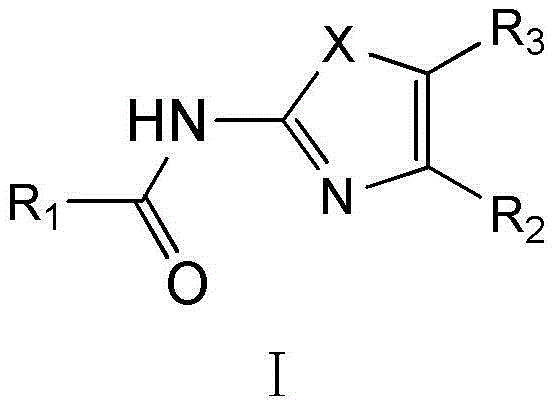
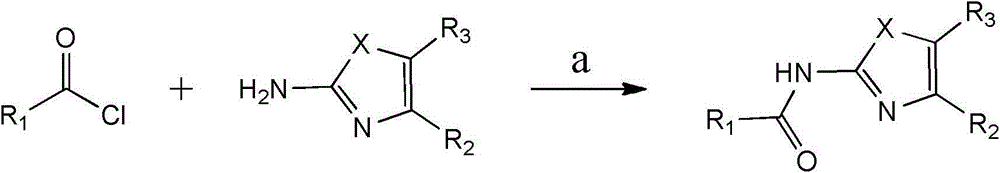
Substituted 1,3-miscellaneous azole compound, preparation method thereof, pharmaceutical composition containing substituted 1,3-miscellaneous azole compound and purpose thereof
ActiveCN105820163AGood choiceAntibacterial agentsOrganic active ingredientsIsoniazidAntituberculous drug
The invention relates to a substituted 1,3-miscellaneous azole compound, a preparation method thereof, a pharmaceutical composition containing the substituted 1,3-miscellaneous azole compound and a purpose thereof. The invention relates to the compound in a formula I, or its pharmaceutically acceptable salt, a stereisomer or a solvate, wherein R1, R2, R3 and X are defined as a specification; and the invention also relates to the preparation method, the pharmaceutical composition containing the substituted 1,3-miscellaneous azole compound and the purpose thereof. The compound of the present invention is the novel antituberculous compound, is effective to mycobacterium tuberculosis-susceptible strains, also possesses activity on strains with tolerance on traditional first-line antituberculous drugs such as isoniazide and rifampicin, and has good selectivity to mycobacterium tuberculosis.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

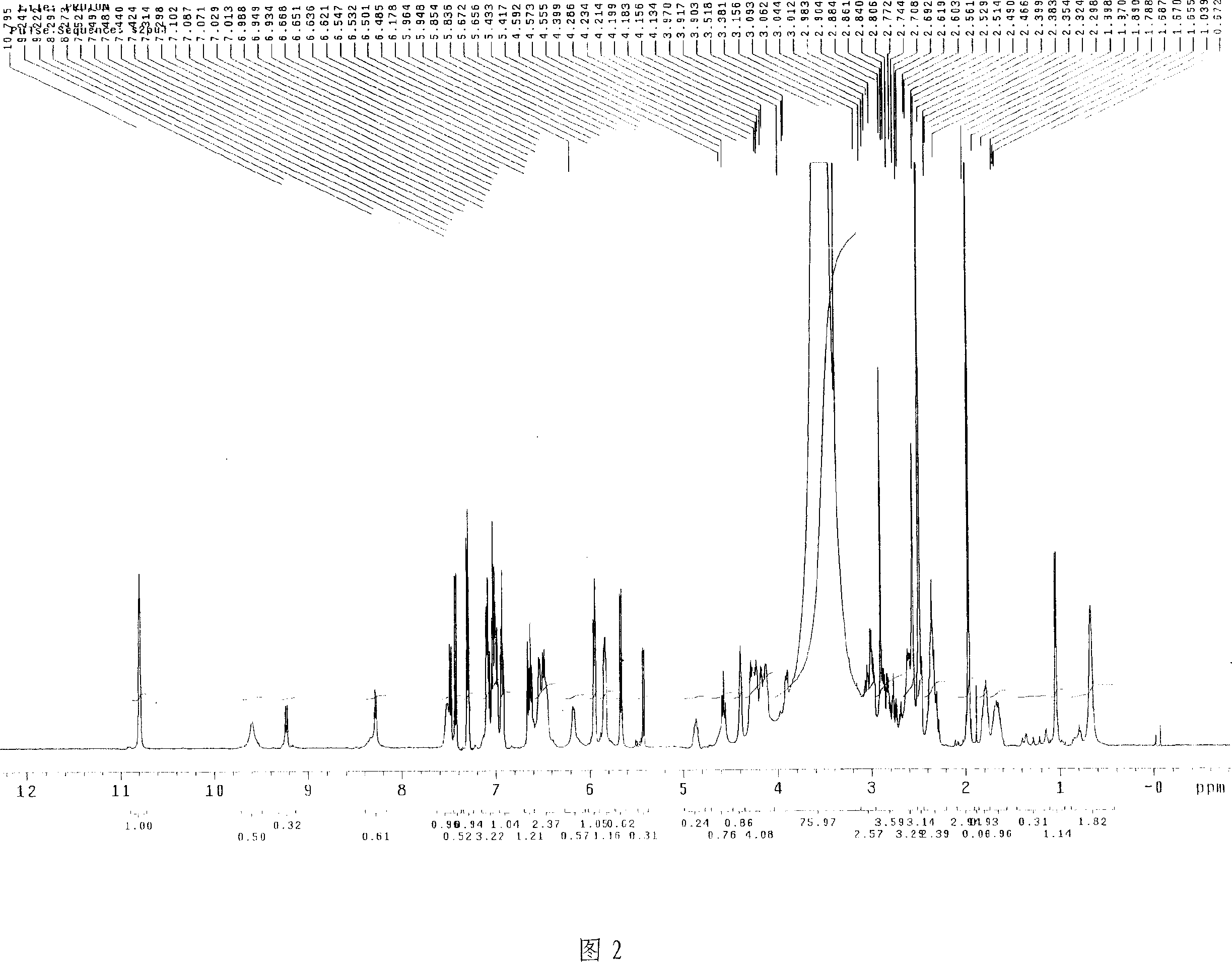
![Pyrazolo[1, 5-a]pyridine compound and use thereof Pyrazolo[1, 5-a]pyridine compound and use thereof](https://images-eureka.patsnap.com/patent_img/909a5abe-a2d3-4ecd-9adb-643e3707a5fe/HDA0000770441560000011.PNG)
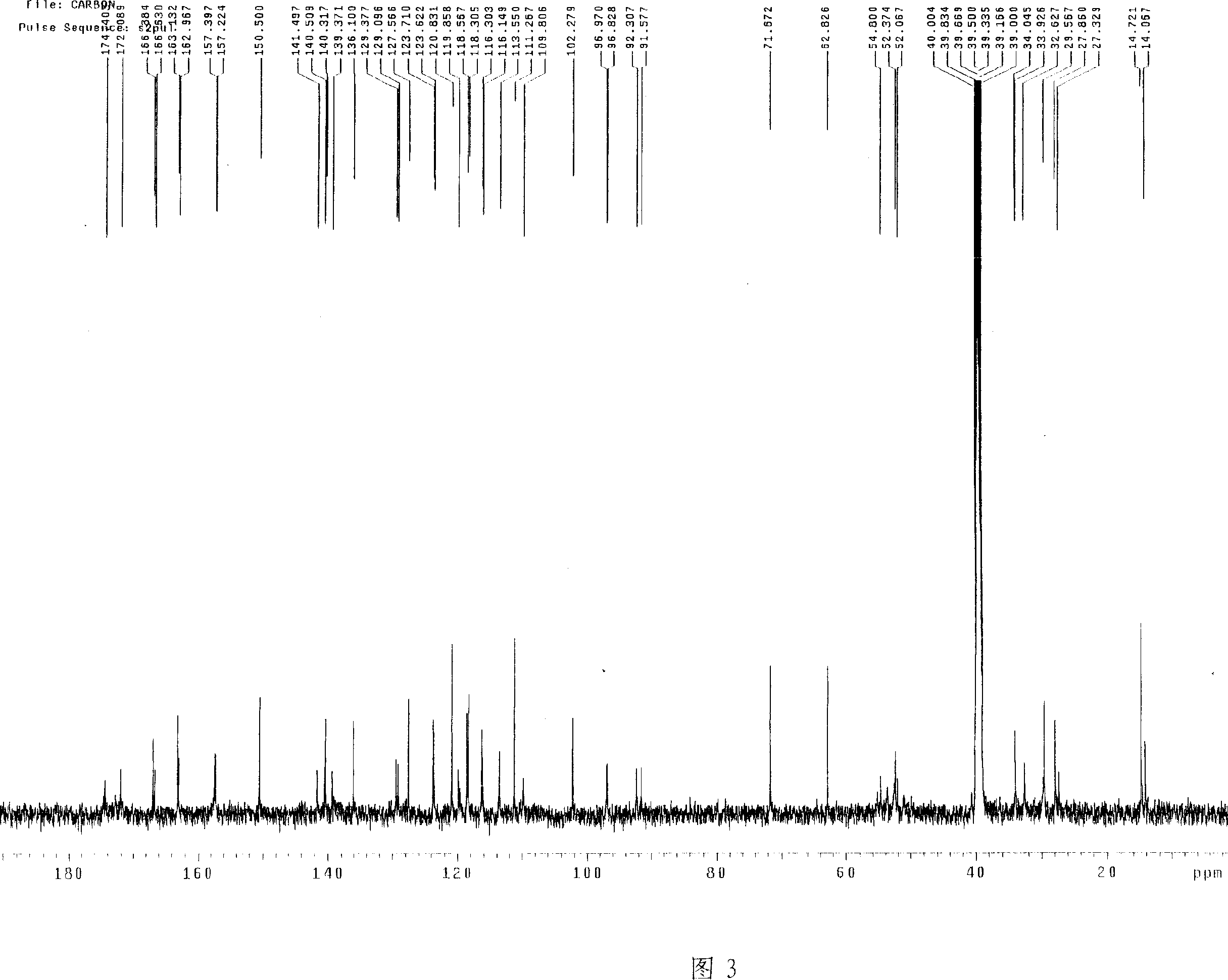
![Pyrazolo[1, 5-a]pyridine compound and use thereof Pyrazolo[1, 5-a]pyridine compound and use thereof](https://images-eureka.patsnap.com/patent_img/909a5abe-a2d3-4ecd-9adb-643e3707a5fe/HDA0000770441560000012.PNG)
![Pyrazolo[1, 5-a]pyridine compound and use thereof Pyrazolo[1, 5-a]pyridine compound and use thereof](https://images-eureka.patsnap.com/patent_img/909a5abe-a2d3-4ecd-9adb-643e3707a5fe/HDA0000770441560000021.PNG)