Reagent for mycobacterium tuberculosis infection detection, clinical treatment effect tracking and antituberculous vaccine development and application thereof
A technology for clinical treatment of Mycobacterium tuberculosis, applied in the field of biomedical testing, can solve the problems that specific immunological detection has not been widely used, false positives, unsatisfactory diagnostic value of tuberculosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Preparation of Mycobacterium tuberculosis Rv3006 antigen and polypeptide.
[0078] 1. The experimental steps for the preparation of Rv3006 fusion protein (antigen) are as follows:
[0079] 1. Use PCR to amplify the Rv3006 gene from Mycobacterium tuberculosis DNA. After sequencing, insert the gene fragment into the E.coli expression plasmid pET28a to construct the pET28a-Rv3006 prokaryotic expression plasmid.
[0080] 2. The pET28a-Rv3006 prokaryotic expression plasmid was transformed into Escherichia coli E.coli BL21(DE3), and the expression engineered bacteria were constructed.
[0081] 3. Use 0.4mM IPTG at 30℃, 220rpm to induce Rv3006 antigen expression for 3h.
[0082] 4. After ultrasonically lysing the bacteria, centrifuge to collect the supernatant protein solution.
[0083] 5. Purify the Rv3006 fusion protein with Ni-NTA magnetic beads.
[0084] 6. The concentration of the fusion protein was detected by the BCA (Pierce, 23227) method, and stored in a...
Embodiment 2
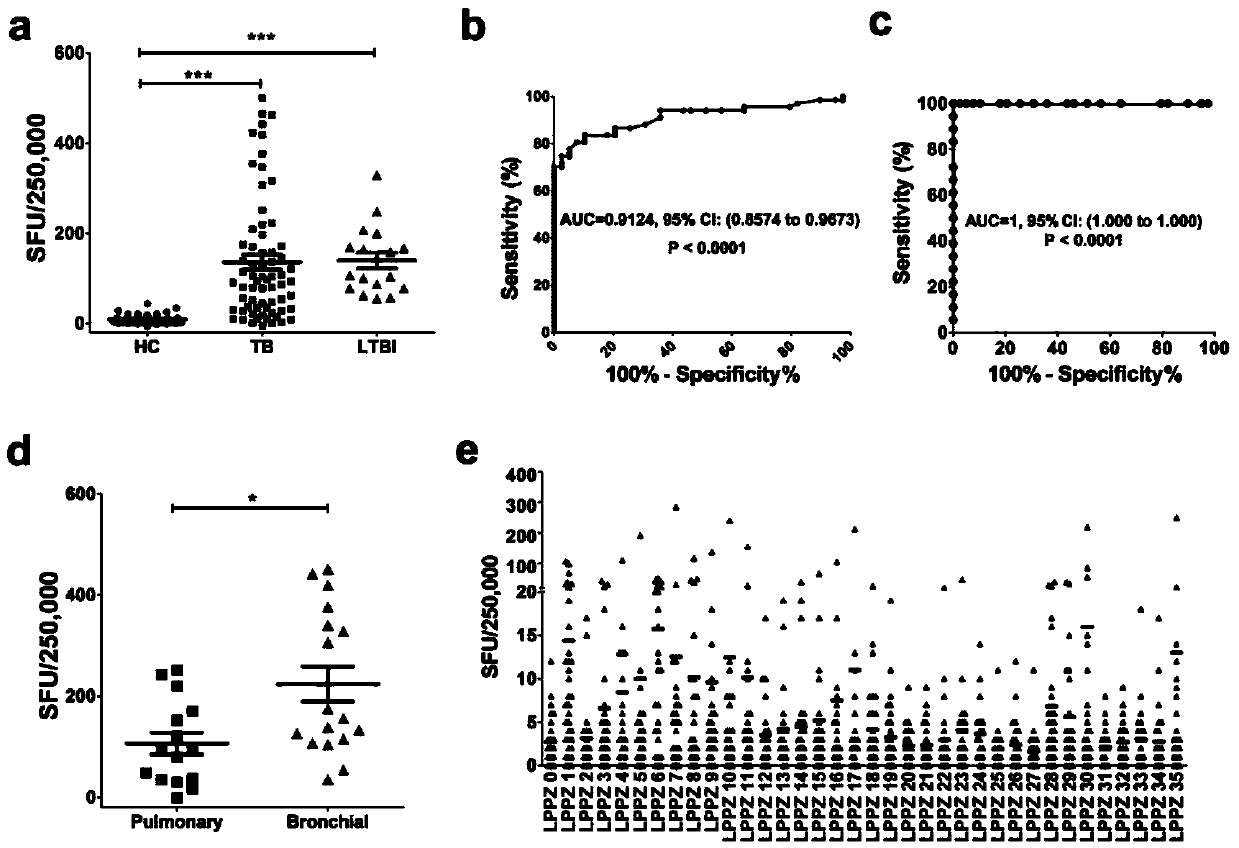
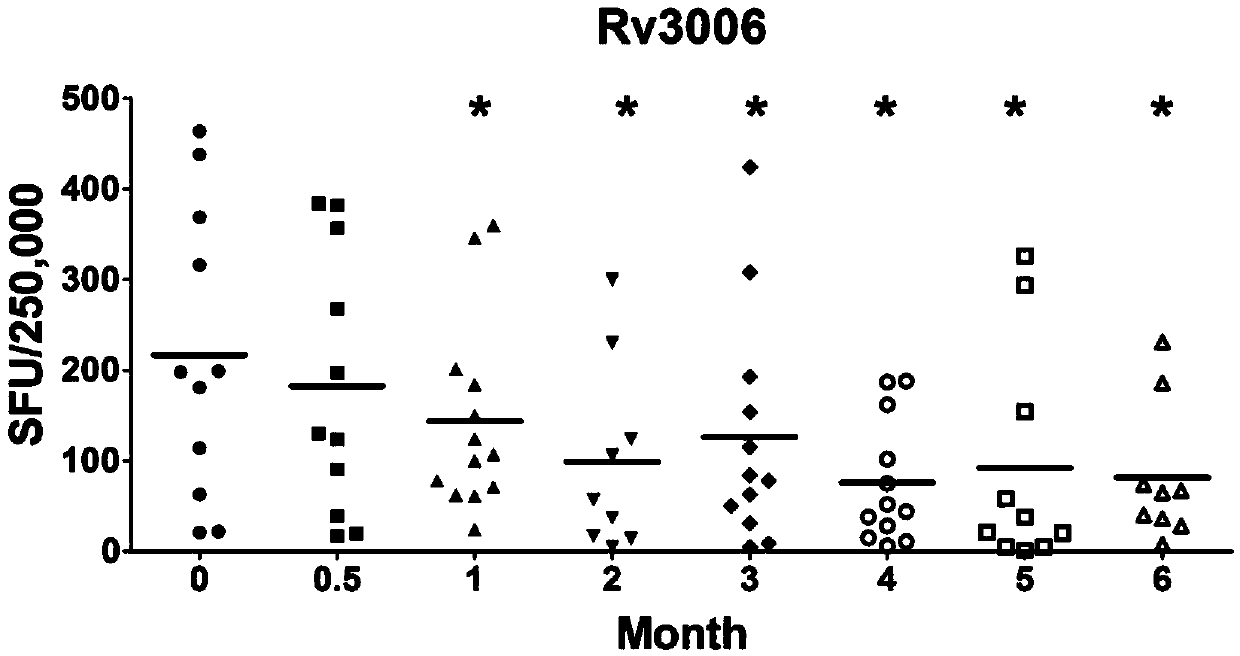
[0124] Example 2: ELISPOT detects the number of specific gamma-interferon-releasing cells stimulated by Rv3006 antigen and its polypeptide
[0125] The experimental steps are as follows:
[0126] 1. Coat ELISPOT 96-well PVDF plate (Millipore MSIPS4510) with IFN-γ antibody, and keep overnight at 4°C.
[0127] 2. Add 200 μl of PBS or RPMI1640 to each well to wash 3 times, and block with RPMI1640 containing 10% FBS at 37° C. for 1 hour.
[0128] 3. Use an anticoagulant tube containing sodium heparin, sodium citrate or dipotassium EDTA to collect about 5 ml from the periphery of tuberculosis patients or normal control populations.
[0129] 4. Using Ficoll density gradient centrifugation method, centrifuge at 1000×g, 18-22°C for 22min. Peripheral blood mononuclear cells (PBMC) were isolated.
[0130] 5. PBMC were washed twice with 5-10ml PBS or RPMI1640 and resuspended in RPMI1640 containing 10% FBS. Calculate the number of cells and adjust to a final concentration of 2.5×10 6 ...
Embodiment 3
[0139] Example 3: ELISA detection of Rv3006-specific antibody levels in the peripheral blood of active tuberculosis patients and normal control populations.
[0140] The experimental steps are as follows:
[0141] 1. 1 μg / ml of Rv3006 fusion protein was coated with ELISA 96-well plate (Corning9018), and kept overnight at 4°C.
[0142] 2. Discard the supernatant, wash each well with 300 μl of PBS (PBST) containing 0.05% Tween-20 5 times, and pat dry each time on absorbent paper.
[0143] 3. Block with PBS containing 1% gelatin at 37°C for 1 hour.
[0144] 4. Add 300 μl PBST to each well to wash 5 times. Add 100 μl of peripheral blood plasma from tuberculosis patients or normal persons diluted 1:100 to each well, and the dilution solution is PBST containing 1% gelatin. Incubate at 37°C for 1 hour.
[0145] 5. Add 300 μl PBST to each well to wash 6 times. Add 100 μl of HRP-labeled anti-human IgG antibody diluted in PBST containing 1% gelatin to each well, and incubate at 37°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com