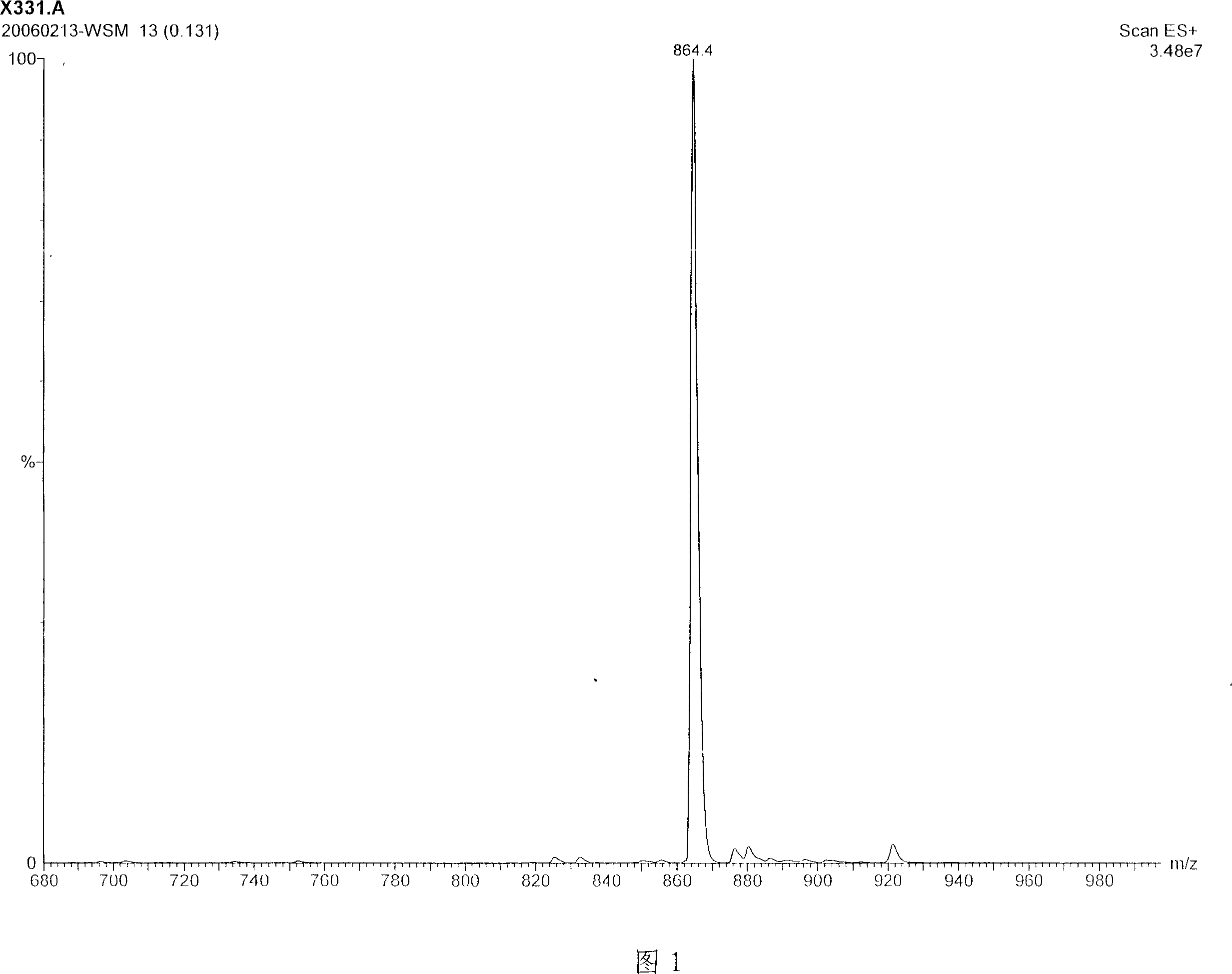
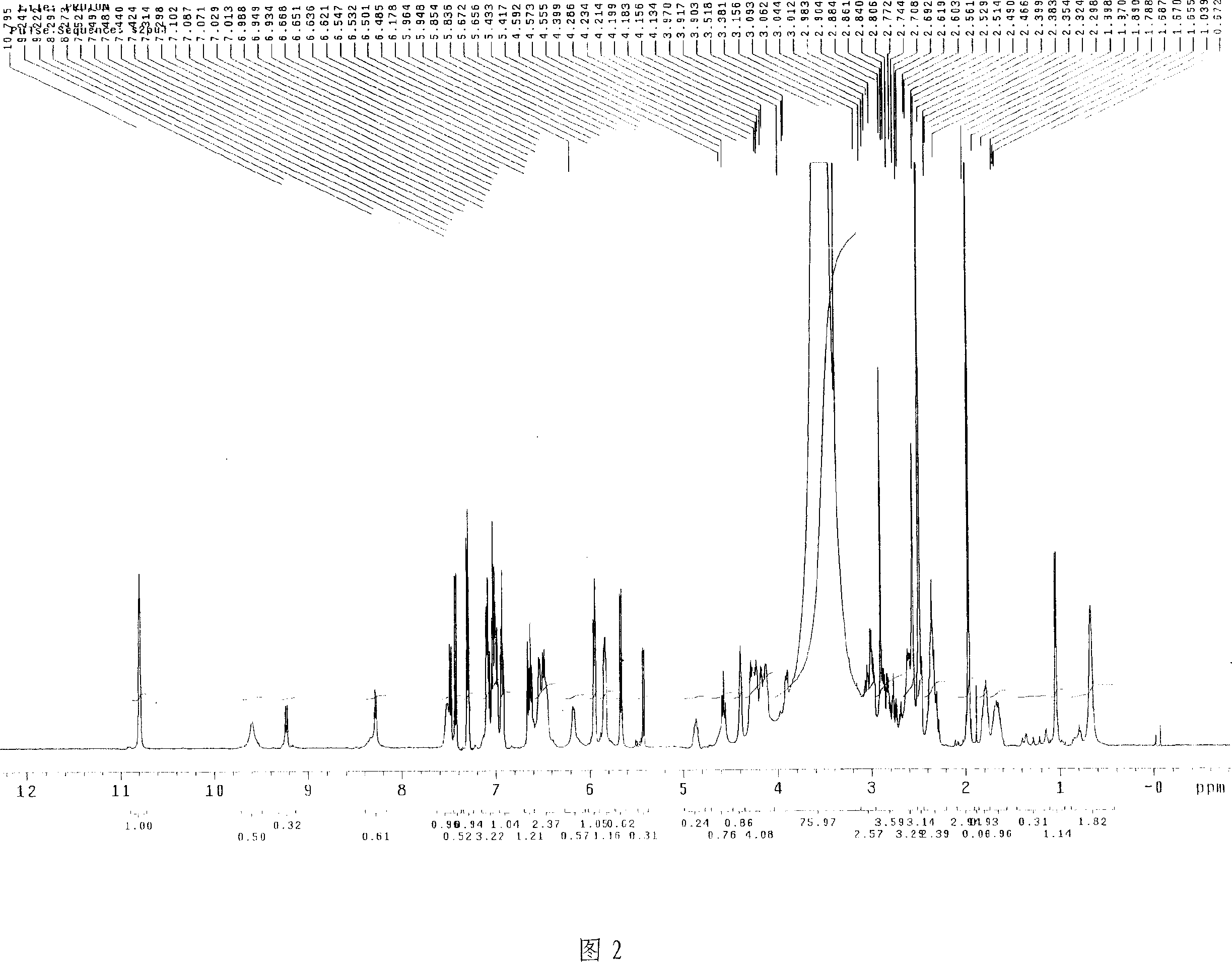
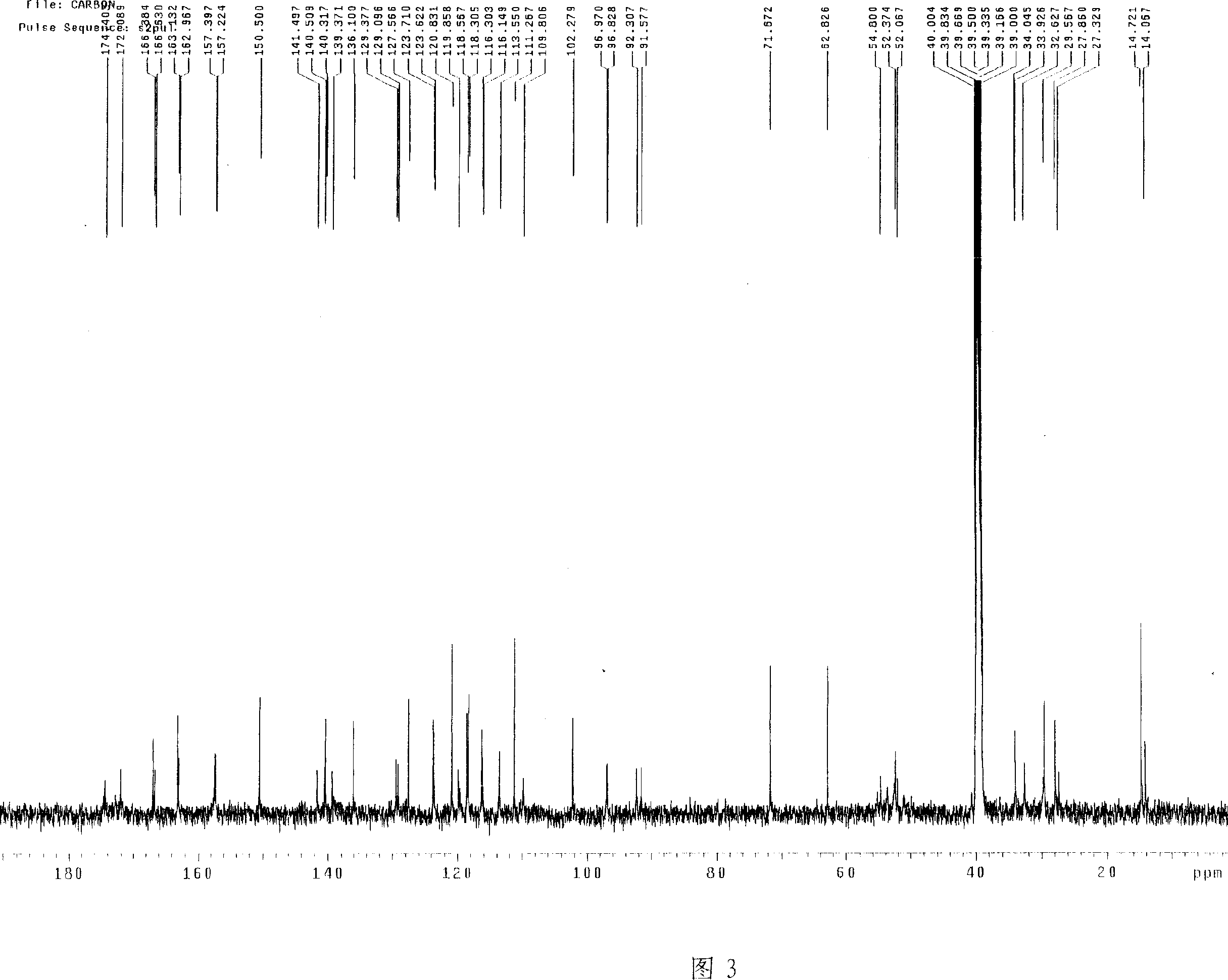
Uridine peptide antibiotic, pharmaceutically acceptable salt, producing method and uses thereof
A technology for uridine peptides and antibiotics, applied in the field of uridine peptide antibiotics and their pharmaceutically acceptable salts, capable of solving the problems of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] From glucose 0.5%; soluble starch 2.4%; beef extract 0.3%; yeast extract 0.5%; peptone 0.5%; corn steep liquor 0.4%; CoCl 2 0.002%; CaCO 3 0.4%, all the other are water, 100 milliliters of culture medium (pH value is 7.2) of composition are filled in the Erlenmeyer flask of 500 milliliters, after sterilization, inoculate Streptomyces SS (Streptomyces SP.) CGMCC No.1764, at 27 ℃ Rotate and shake (220 times / min, amplitude 7cm) for 2 days and use as seeds.
[0063] Then glucose 0.5%; yeast extract 0.5%; peptone 0.5%; beef extract 0.5%; corn steep liquor 0.4%; soybean meal 1%; starch 2%; 3 0.4%; CoCl 2 ·6H 2 0.4‰, all the other are water, form fermented medium (pH value is 7.2), sub-pack 100 milliliters in the Erlenmeyer flask of 500 milliliters, add the seed of 2% above-mentioned cultivation after sterilization, at 27 ℃, rotary oscillation ( 220 times / min, amplitude 7cm) cultivated for 4 days to obtain the fermented liquid.
[0064] Take the fermentation broth (20 lit...
Embodiment 2
[0102] Basically the same as Example 1, the difference is that the fermentation medium is: 0.4% glucose; 0.4% yeast extract; 0.6% peptone; 0.6% beef extract; 0.3% corn steep liquor; 3 0.5%; CoCl 2 ·6H 2 O0.3‰, the rest is water, and the pH value is 7.2.
[0103] The crude product containing SS-1, SS-2 and SS-3 obtained by fermentation is separated, extracted and refined to obtain antibiotics containing SS-1, SS-2 and SS-3. Then use neutralization reaction to make ammonia salt. Then 50 grams of its ammonium salt and 100 grams of base semi-synthetic glyceride are heated and mixed to be placed in a mould, and made into a suppository after cooling.
Embodiment 3
[0105] Basically the same as Example 1, the difference is that the fermentation medium is: 0.6% glucose; 0.6% yeast extract; 0.4% peptone; 0.4% beef extract; 0.5% corn steep liquor; 3 0.3%; CoCl 2 ·6H 2 O0.5‰, the rest is water, and the pH value is 7.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com