Synthetic method of anti-tuberculosis candidate drug PA-824
A technology of PA-824 and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high price, achieve the effect of easy to obtain raw materials, avoid low temperature reaction, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
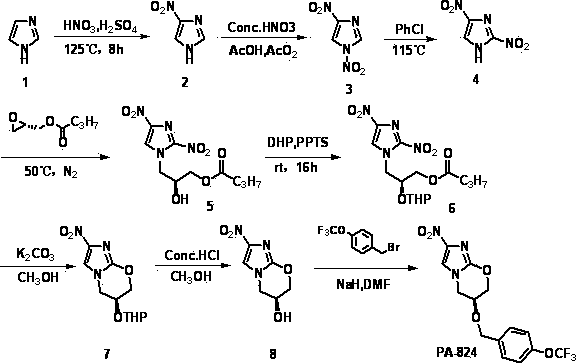
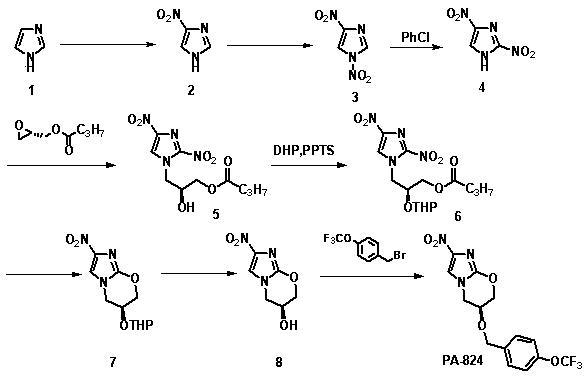
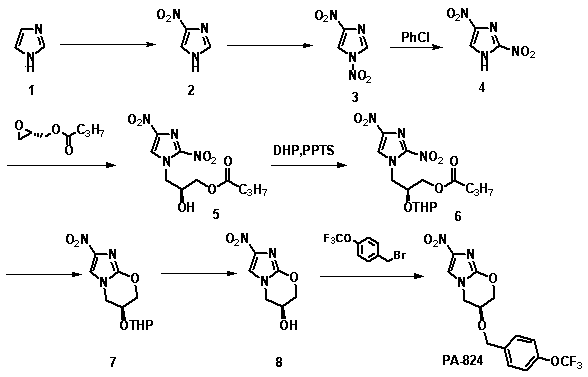
[0026] The synthetic method of nitroimidazole pyran class anti-tuberculosis drug candidate PA-824 comprises the following steps:
[0027]
[0028] (1) In concentrated H 2 SO 4 In, compound 1 was mixed with 4 times concentrated HNO 3 The reaction is nitroated to obtain compound 2;
[0029] (2) In acetic acid and acetic anhydride, compound 2 was reacted with fuming nitric acid for re-nitration to obtain compound 3;
[0030] (3) Compound 3 undergoes thermal rearrangement in chlorobenzene to obtain compound 4;
[0031] (4) Compound 4 and ( S )-(+)-glycidyl butyrate undergoes a nucleophilic ring-opening reaction to obtain compound 5;
[0032] (5) in CH 2 Cl 2 Among them, compound 5 and 3,4-dihydropyran protect the hydroxyl group under the action of catalyst PPTS to obtain compound 6;
[0033] (6) in CH 3 In OH, compound 6 is in an equimolar amount of K 2 CO 3 Under the action of ester hydrolysis and intramolecular nucleophilic ring closure reaction, compound 7 was obt...
Embodiment 1
[0045] compound 2 preparation of
[0046] Add 320 ml of concentrated H to a 2000 ml three-neck flask 2 SO 4 , slowly add 100 g of imidazole with stirring in an ice bath, continue to stir after the addition, and after the solution becomes clear, slowly add 240 ml of concentrated HNO 3 . After dripping, raise the temperature to 125 °C for reflux reaction for 10 h, stop the reaction, cool to room temperature, pour into about 10 times of crushed ice, a large amount of white solid precipitates, filter with suction, wash with cold water, and dry the filter cake to obtain the product 4 -Nitroimidazole 155g, the filtrate was adjusted to pH 3-4 with concentrated ammonia water, and the solid was precipitated, suction filtered, washed with cold water, and the filter cake was dried to obtain 13 g of the product 4-nitroimidazole, and a total of 178 g of the product was obtained. 85.04%. 1 H-NMR (400M, d6-DMSO): δ 7.85 (s, 1H), 8.32 (s, 1H).
[0047] compound 3 preparation of
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com