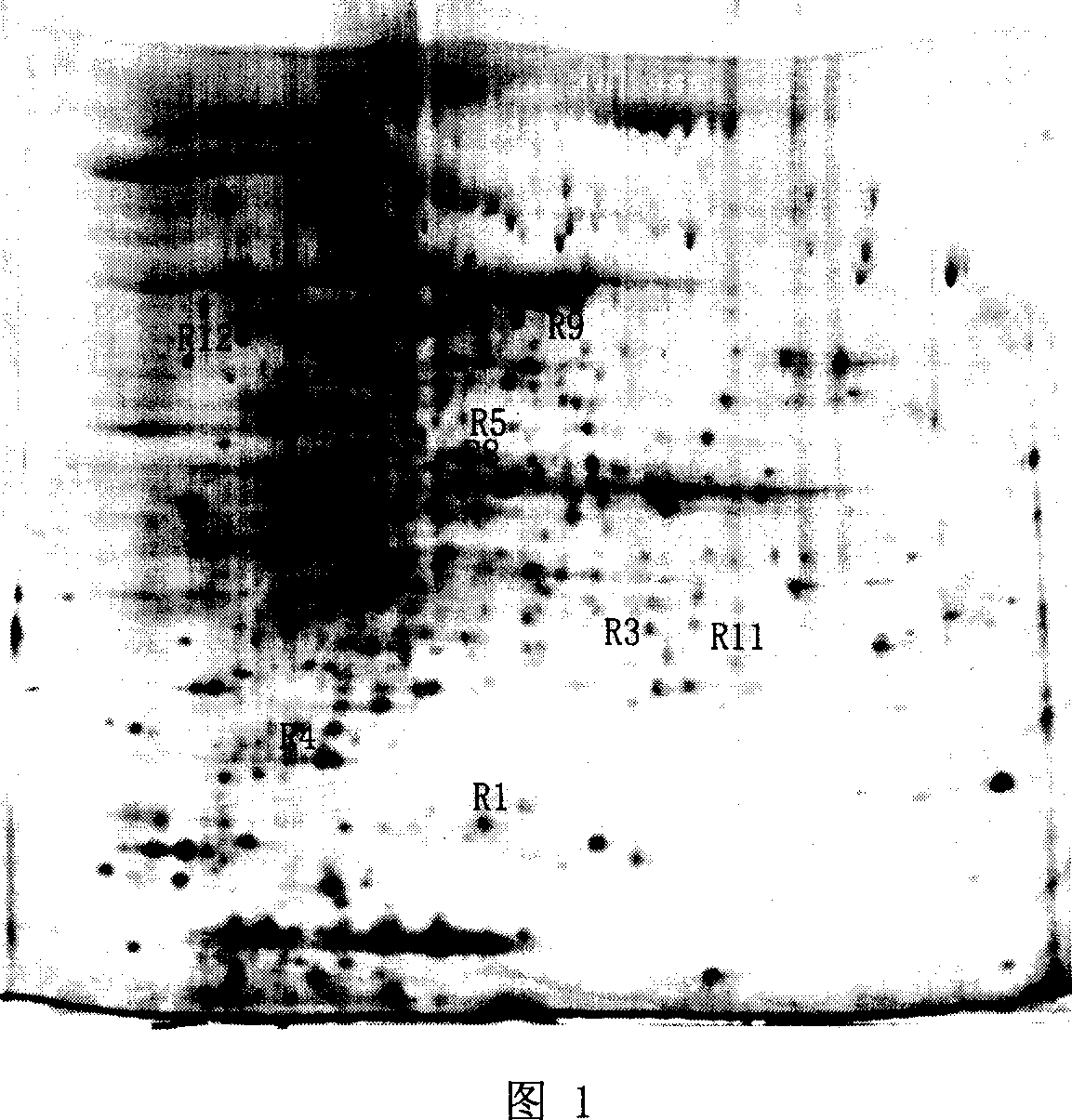
Method for screening Mycobacterium tuberculosis drug-resistant protein
A Mycobacterium tuberculosis and protein technology, which is applied in the fields of biochemical equipment and methods, microbial determination/inspection, material inspection products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The cultivation of embodiment 1 Mycobacterium tuberculosis and drug susceptibility test
[0037] Mycobacterium tuberculosis standard strain Mycobacterium tuberculosis H37Rv (ATCC93009), clinical rifampicin (ie for 3-{[(4-methyl-1-piperazinyl)imino]methyl}-rifamycin, rifampicin , RFP, Sigma R 3501) single-drug-resistant strains (5 strains), and clinical all-sensitive strains (9 strains) were provided by the Tuberculosis Laboratory of Shanghai Pulmonary Hospital. The strains were inoculated into Middlebrook 7H9Broth (DIFCO, Bection-Dickinson) added with 10% ADC (DIFCO, 0.5% bovine serum albumin, 0.2% glucose, 140mmol / LNaCl) and 0.5% glycerol, and placed in a static incubator at 37°C. Place and cultivate for 15-20 days, when the bacterial concentration reaches to 1×10 8 ~2×10 8 or optical density (OD 600 ) was collected between 0.8 and 1.
[0038] Heat in a water bath at 80°C for 2 hours to kill bacteria. After centrifugation at 5000rpm at 4°C, the supernatant was dis...
Embodiment 2
[0040] The extraction of embodiment 2 thalline protein
[0041] Centrifuge at 6000g at 4°C for 15 minutes to precipitate 20 mL of cells in logarithmic growth phase. The pelleted bacteria were washed twice with PBS buffer at pH 7.4. Dissolve the bacteria with 0.5mL sonication buffer. Sonicate in an ice bath to disrupt and lyse bacteria for 15 minutes, with an amplitude of 70%, 200W for 1 minute, and stop for 20 seconds. Slowly add sample lysate and place at room temperature for 30 minutes, shaking at intervals. Centrifuge at 10,000 r / min at 20°C for 15 minutes, collect the supernatant, and freeze at -80°C. Protein concentration was measured by Bradford method.
Embodiment 3
[0042] The extraction of embodiment 3 basic protein
[0043] Centrifuge at 6000g at 4°C for 15 minutes to precipitate 20 mL of cells in logarithmic growth phase. Centrifuge at 4000r / min at 4°C for 15 minutes to precipitate the bacteria, and wash the precipitated bacteria twice with PBS buffer solution of pH 7.4. Resuspend the pelleted bacteria with 1 mL of LPBS and transfer to a 1.5 mL EP tube. Centrifuge at 6000r / min for 15 minutes at 4°C to pellet the bacteria. Dissolve the bacteria with 0.5mL sonication buffer. Sonicate in an ice bath to disrupt and lyse bacteria for 15 minutes, with an amplitude of 70%, 200W for 1 minute, and stop for 20 seconds. Fix with pre-cooled methanol at 4°C for 40 minutes (change methanol once in the middle), slowly add 1 / 4 volume of pre-cooled 1.0N H 2 SO 4 , stirring continuously for 30 minutes. The suspension was centrifuged at 12000×g for 20 minutes at 4°C, and the supernatant was retained. Break up the pellet and resuspend in cold 0.4N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com